-
PDF
- Split View
-
Views
-
Cite
Cite
Omar AlShalabi, Bayan Alsaid, Abdulghani AlShalabi, A huge extrarenal cell carcinoma developing from a heterotopic renal anlage with distant metastases: case report and literature review, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa227, https://doi.org/10.1093/jscr/rjaa227
Close - Share Icon Share
Abstract
Renal cell carcinoma (RCC) is the most common type of renal tumor arising from the proximal renal tubules. Extrarenal RCC is a rare case in which a tumor is found outside the kidney with no primary kidney tumor. Some theories suggest that these tumors arise from mesoderm remnant. Here, we present a unique case, the fourth in literature, of peri-renal, Extrarenal RCC case and the first case with a huge mass with distant metastases and aggressive progression.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of renal tumor arising from the proximal renal tubules [1]. Extrarenal RCC is a rare case in which a tumor is found outside the kidney with no primary kidney tumor. Some theories suggest that these tumors arise from mesoderm remnant [2], renal anlage. Here, we present a case of an extrarenal clear cell RCC with metastases to surrounding lymph nodes and lung. After an extensive review of the literature and to our knowledge, we are presenting the fourth, peri-renal, Extrarenal RCC case and the first case with a huge mass with distant metastases.
CASE PRESENTATION
A 62-year-old woman was admitted to our hospital complaining of fatigue, general weakness, anorexia and weight loss (about 8 kg in the past 3 months). The patient did not suffer of any pain; no gastroenterological or urological symptoms were reported. Her past medical history showed controlled hypertension and surgical salpingo-hysterectomy. Physical examination showed a hard palpable left upper quadrate mass, with skin movable upon the mass, and deep structure fixation.
Abdominal ultrasound showed a 16 cm mass in the left adrenal position, abutting the spleen, left kidney and pancreas. A whole body computed tomography (CT) scan showed: a (14.5 × 9.8 cm) well circum heterogeneously enhancing soft tissue mass, pressing the spleen and pancreatic body and tail, the mass seemed to be in the left adrenal vicinity did not extend to the midline. Both kidneys and urinary systems showed no abnormalities (Fig. 1). The right lobe of the liver showed two nodular lesions, 7 and 5 mm, suggesting hemangiomas. No adenopathy in retroperitoneal space was noticed. A positron emission tomography (PET) scan was obtained, and it showed the same primary huge mass uptaking fluorodeoxyglucose, confirming the carcinogenic origin with no distant metastases at that time. Colonoscopy revealed a mass effect at splenic flexure, with no other abnormalities till 20 cm of the ileum.
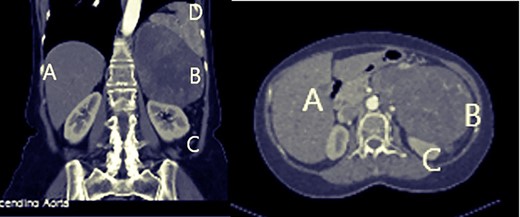
Abdominal computed tomography: A: liver, B: mass, C: left kidney.
Blood tests were remarkable for minor anemia (Hg: 10.4 g/dl), with elevation in lactate dehydrogenase (501 U/l), C-reactive protein (64.9 mg/dl) and erythrocyte sedimentation rate in the first hour (73 mm/h). Regarding the endocrinology study, blood cortisol levels monitored in mornings and nights were within normal limits. No abnormalities were noticed in metanephrine, normetanephrine and vanillylmandelic acid (VMA) levels in 24-h urine collection sample. Blood sugar ranged between 148 and 98 mg/dl. Values of her arterial blood pressure presurgery ranged between systolic (12–16 mmHg) and diastolic (7–9 mmHg).
Upon laparotomy, a left subcostal incision was used to access the abdomen. The mass was found in the left adrenal vicinity with no connection to adjacent structures (Fig. 2B). It was easily enucleated and isolated from the spleen, pancreas and kidney. Its vascular peduncle was identified and ligated (Fig. 2D). The kidney was found to be completely intact upon inspection and was left in place.
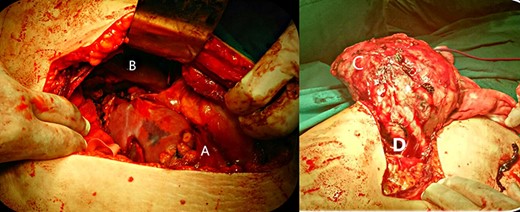
Surgical operation. A: mass, B: liver, C: enucleated mass, D: vascular peduncle.
Post-surgical period was uneventful, and the patient was discharged 5 days after surgery with no complications.
Histologically, the mass was well-delineated unencapsulated, measuring 16 cm× 15.5 cm × 15 cm, weighting 1096 g. Cross sections revealed fleshy hemorrhagic and necrotic tissues. Microscopic examination showed proliferation of large clear cell with fine lobulation and increased mitotic activity, which consists of clear cell carcinoma grade III. Line of surgical resection is free and morphologically was typical of renal origin. No normal renal or adrenal gland was noticed.
Immunohistochemical (IHC) study was performed with the following results: VIMENTIN, epithelial membrane antigen (EMA) and neutral endopeptidase CD10 were positive, whereas cytokeratin (CK), CK20 and CK07 were negative. Depending on all previous information, the mass most consistent with clear cell carcinoma is renal/renal anlage.
A PET scan was obtained 6 weeks after the surgery, and it showed metastases to lymph nodes of the left kidney hilum and lungs. A few days following the second chemotherapy dose, the patient developed severe ascites, pulmonary effusion and severe heart failure, which developed into multi-organ failure, and the patient passed away 3 months after the surgery.
LITERATURE REVIEW
Beside our case, three cases of Extrarenal RCC were reported, and data were summarized in Table 1.
| . | Age, years . | Sex . | Country . | Position . | Volume . | IHC . | Mets . |
|---|---|---|---|---|---|---|---|
| Terada [2] | 83 | Male | Japan | Near the lower pole of the right kidney, pedunculated RCC | A small tumor 1 × 1× 1 cm | (+): RCC ma, CD10, CD15, cytokeratin CAM5.2, CK AE1/3, CK18, AMACR and Ki-67 | No |
| (−): CK8, HMB45, inhibin, calretinin, CD68, p53, TTF-1, and CDX2 | |||||||
| Hasan et al. [3] | 28 | Female | India | Arising from left adrenal gland | 8 cm × 7.5 cm ×5.5 cm | (+): CD10, weakly CK | No |
| (−): Chromogranin, EMA and inhibin | |||||||
| Al-Maghrebi and Khabaz [4] | 37 | Male | Saudi Arabia | Right retroperitoneal soft tissue mass | 7.9 × 5.3 × 12.6 cm | (+): AMACR, PAX-8, CD10, RCC ma and TFE3 | Not mentioned |
| (−): CK AE1/AE3, CK 7, CK 8/18, vimentin, HMB-45, desmin, SMA, EMA, and MSA | |||||||
| Xp11.2 translocation-associated RCC | |||||||
| Our case | 62 | Female | Syria | Left adrenal vicinity | 16 cm× 15.5 cm × 15 cm | (+): VIMENTIN, EMA and CD10 | Yes |
| (−): CK, CK20 and CK07 |
| . | Age, years . | Sex . | Country . | Position . | Volume . | IHC . | Mets . |
|---|---|---|---|---|---|---|---|
| Terada [2] | 83 | Male | Japan | Near the lower pole of the right kidney, pedunculated RCC | A small tumor 1 × 1× 1 cm | (+): RCC ma, CD10, CD15, cytokeratin CAM5.2, CK AE1/3, CK18, AMACR and Ki-67 | No |
| (−): CK8, HMB45, inhibin, calretinin, CD68, p53, TTF-1, and CDX2 | |||||||
| Hasan et al. [3] | 28 | Female | India | Arising from left adrenal gland | 8 cm × 7.5 cm ×5.5 cm | (+): CD10, weakly CK | No |
| (−): Chromogranin, EMA and inhibin | |||||||
| Al-Maghrebi and Khabaz [4] | 37 | Male | Saudi Arabia | Right retroperitoneal soft tissue mass | 7.9 × 5.3 × 12.6 cm | (+): AMACR, PAX-8, CD10, RCC ma and TFE3 | Not mentioned |
| (−): CK AE1/AE3, CK 7, CK 8/18, vimentin, HMB-45, desmin, SMA, EMA, and MSA | |||||||
| Xp11.2 translocation-associated RCC | |||||||
| Our case | 62 | Female | Syria | Left adrenal vicinity | 16 cm× 15.5 cm × 15 cm | (+): VIMENTIN, EMA and CD10 | Yes |
| (−): CK, CK20 and CK07 |
RCC ma, RCC marker; AMACR, a-methyl CoA racemase.
| . | Age, years . | Sex . | Country . | Position . | Volume . | IHC . | Mets . |
|---|---|---|---|---|---|---|---|
| Terada [2] | 83 | Male | Japan | Near the lower pole of the right kidney, pedunculated RCC | A small tumor 1 × 1× 1 cm | (+): RCC ma, CD10, CD15, cytokeratin CAM5.2, CK AE1/3, CK18, AMACR and Ki-67 | No |
| (−): CK8, HMB45, inhibin, calretinin, CD68, p53, TTF-1, and CDX2 | |||||||
| Hasan et al. [3] | 28 | Female | India | Arising from left adrenal gland | 8 cm × 7.5 cm ×5.5 cm | (+): CD10, weakly CK | No |
| (−): Chromogranin, EMA and inhibin | |||||||
| Al-Maghrebi and Khabaz [4] | 37 | Male | Saudi Arabia | Right retroperitoneal soft tissue mass | 7.9 × 5.3 × 12.6 cm | (+): AMACR, PAX-8, CD10, RCC ma and TFE3 | Not mentioned |
| (−): CK AE1/AE3, CK 7, CK 8/18, vimentin, HMB-45, desmin, SMA, EMA, and MSA | |||||||
| Xp11.2 translocation-associated RCC | |||||||
| Our case | 62 | Female | Syria | Left adrenal vicinity | 16 cm× 15.5 cm × 15 cm | (+): VIMENTIN, EMA and CD10 | Yes |
| (−): CK, CK20 and CK07 |
| . | Age, years . | Sex . | Country . | Position . | Volume . | IHC . | Mets . |
|---|---|---|---|---|---|---|---|
| Terada [2] | 83 | Male | Japan | Near the lower pole of the right kidney, pedunculated RCC | A small tumor 1 × 1× 1 cm | (+): RCC ma, CD10, CD15, cytokeratin CAM5.2, CK AE1/3, CK18, AMACR and Ki-67 | No |
| (−): CK8, HMB45, inhibin, calretinin, CD68, p53, TTF-1, and CDX2 | |||||||
| Hasan et al. [3] | 28 | Female | India | Arising from left adrenal gland | 8 cm × 7.5 cm ×5.5 cm | (+): CD10, weakly CK | No |
| (−): Chromogranin, EMA and inhibin | |||||||
| Al-Maghrebi and Khabaz [4] | 37 | Male | Saudi Arabia | Right retroperitoneal soft tissue mass | 7.9 × 5.3 × 12.6 cm | (+): AMACR, PAX-8, CD10, RCC ma and TFE3 | Not mentioned |
| (−): CK AE1/AE3, CK 7, CK 8/18, vimentin, HMB-45, desmin, SMA, EMA, and MSA | |||||||
| Xp11.2 translocation-associated RCC | |||||||
| Our case | 62 | Female | Syria | Left adrenal vicinity | 16 cm× 15.5 cm × 15 cm | (+): VIMENTIN, EMA and CD10 | Yes |
| (−): CK, CK20 and CK07 |
RCC ma, RCC marker; AMACR, a-methyl CoA racemase.
DISCUSSION
RCC is the most common type of renal tumor arising from the proximal renal tubules [1]. Extrarenal RCC refers to the occurrence of RCC in locations other than the normal native kidneys, likely arising from mesonephric remnants that have been mentioned twice in previous literature [2, 3].
The possibility that this tumor might not be an Extrarenal RCC tumor was strongly considered. Secreting adrenal tumors (pheochromocytoma and secreting adrenal cortical tumors) were ruled out due to normal arterial tension values, normal blood and urine cortisol levels, normal blood potassium and sugar levels and normal urine metanephrine, normetanephrine and VMA levels. Our mass showed positivity for EMA, vimentin and neutral endopeptidase CD10, whereas CK, CK20 and CK07 were negative, while adrenal carcinomas and adrenal cortical adenomas are both negative for vimentin and EMA excluding the possibility of our mass being an adrenal carcinoma or an adrenal cortical adenoma [5, 6]. Supernumerary kidney was excluded because no normal kidney tissue was found in the mass, and the possibility of the presented tumor being aroused from a minute ectopic kidney and totally involved it was denied due to the fact that neither a different urinary nor an arterial system were found for the mass.
On embryogenic level, during the fetal kidney development, pronephros, mesonephros and metanephros appear, the former two disappear and the latter one (metanephros) gives rise to metanephric blastema, which lead to the normal kidney [2]. We speculate that during these processes, these mesonephric structures (renal anlage) were persistent in the fetal and postnatal life in this patient, giving rise to Extrarenal RCC and developing till reaching that huge size with distant metastases.
Extrarenal RCC is a sporadic tumor which can affect any age during adulthood, with no specific ethnicity. According to current cases, the reported position in peri-renal area was left gland vicinity in females and around right kidney in men. Its volume can differ from small with high rate of remission to huge tumors with metastases and an aggressive progression. Lung metastasis and local regional lymph nodes are the most common sites of distant metastases. Diagnosing such type of tumor can be a bit challenging since a broad spectrum of lesions share the same position, radiologic and histological characteristics, where IHC study can be helpful. According to recent studies, cell marque cytokeratin CAM 5.2, AE1/AE3, vimentin, EMA, CD10 and RCC marker are the most common positive tests to confirm Extrarenal RCC, whereas inhibin, human melanoma black HMB45 and chromogranin negativity are the most commonly used tests to rule out adrenal lesions [5].
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interests regarding the publication of this paper.



