-
PDF
- Split View
-
Views
-
Cite
Cite
Shane Irwin, Noel Edward Donlon, Helen Mohan, John V Reynolds, Gastro-cholecysto-colic fistula. Case report of an idiopathic case, and management approach, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa162, https://doi.org/10.1093/jscr/rjaa162
Close - Share Icon Share
Abstract
A 71-year-old lady presented with a 4-week-history of epigastric pain, feculent vomiting, diarrhoea and weight-loss. On subsequent investigations, she was found to have a complex gastro-cholecysto-colic fistula with no clear underlying aetiology. The only abnormality both macroscopically and microscopically was ulceration and inflammation in the colon. However, this was not pathognomonic of inflammatory bowel disease, and (gastric) acid-induced inflammation is an alternative explanation. Herein we present her case, her comprehensive evaluation, her successful surgical management and a review of the relevant literature.
INTRODUCTION
Gastrocolic fistula is a pathological connection between the stomach and colon. These can be associated with a variety of diseases including malignancy of the stomach or colon, and benign pathologies including peptic ulcer disease and inflammatory bowel disease (IBD). A cholecysto-enteric fistula usually relates to a large gallstone eroding into the gastrointestinal tract, most typically via the duodenum. Herein we present a highly unusual case of a fistula involving stomach, colon and gallbladder.
CASE REPORT
A 71-year-old female presented to the Emergency Department with a 4-week history of worsening epigastric pain, feculent vomiting and profuse diarrhoea after eating. There was associated weight loss of 10 kg, amounting to 15% of body weight. She had no relevant past medical history, such as peptic ulcer disease, colonic disease or biliary symptoms, or prior surgery. She had been on no medication. She had a 50 pack-year smoking history.
Computed tomography (CT) revealed a complex fistulous communication between the distal stomach and biliary tree with associated pneumobilia, and between the transverse colon and the distal stomach (Fig. 1). No gallstones were seen. Upper and lower gastrointestinal endoscopy was performed, revealing a prepyloric gastric ulceration and fistula (Fig. 2), with no obvious gastric pathology, which extended into the colon and a blind end structure assumed to be the gallbladder. Colonoscopy demonstrated an area of slight narrowing from the hepatic flexure to mid-transverse colon, with macroscopic colitis (Fig. 3). Biopsies were non-specific, with IBD a possibility, but the features were not pathognomonic.
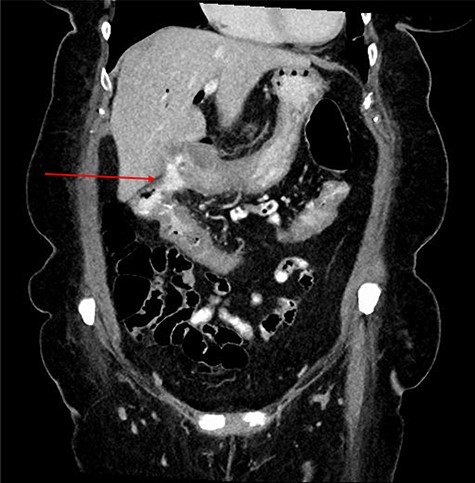
CT abdomen and pelvis demonstrating fistulous communication between distal stomach and transverse colon (red arrow) with pneumobilia.
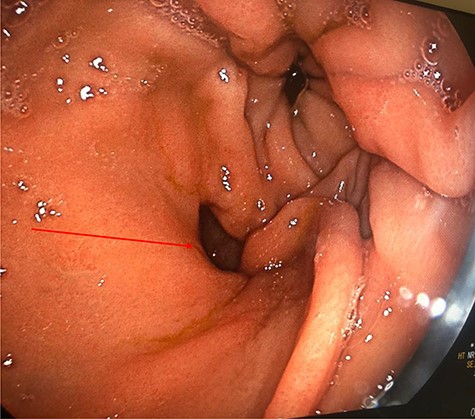
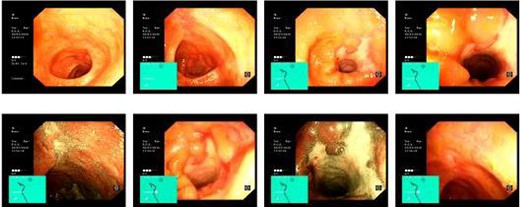
Lower GI endoscopy demonstrating ulceration, stenosis and stricturing of the transverse colon.
To further characterize, an endoscopic ultrasound demonstrated a fistula between the distal antrum and gallbladder containing air and possibly some tiny calculi or sediment. The common bile duct was of normal diameter. There was no lymphadenopathy and no obvious malignancy seen. Magnetic resonance imaging of the small bowel outlined the complex fistula, and non-specific changes in the colon, but provided no further information regarding aetiology (Fig. 4).
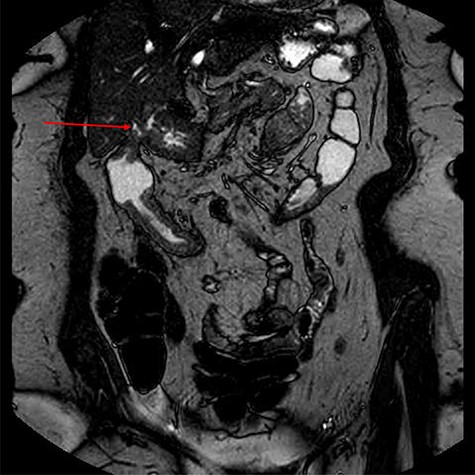
The patient was commenced on total parenteral nutrition for 10 days prior to surgery. At laparotomy, the fistulous mass involving the distal stomach, proximal transverse colon and a shrunken gallbladder with no obvious calculi, was dissected en bloc and resected (Fig. 5). This involved a right hemicolectomy, cholecystectomy and distal gastrectomy, with Roux-en-Y reconstruction to the stomach and an ileo-transverse anastomosis.
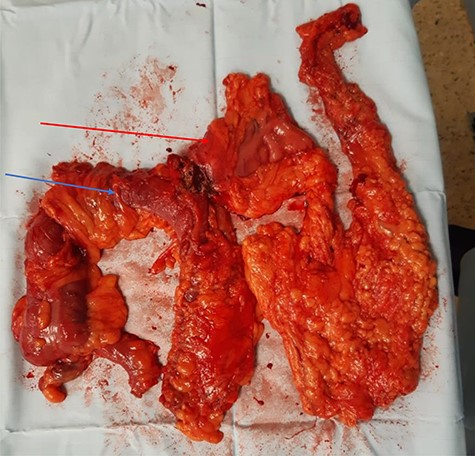
Specimen demonstrating en bloc distal gastrectomy (red arrow), cholecystectomy (shrunken and fibrosed to gastric antrum) and extended right hemicolectomy (blue arrow).
Histology of the distal stomach showed intestinal metaplasia with no dysplasia, tumour or H pylori colonization. Colonic histology showed occasional transmural lymphoid aggregates without definite granulomas suggestive of colonic origin, but definitive histological diagnostic criteria for Crohn’s disease were not met. The gallbladder revealed chronic inflammation, no calculi were evident.
The patient had an uncomplicated postoperative course and reported excellent health 1 month post-operatively.
DISCUSSION
This case is highly unusual in that there is both no clear underlying cause, and in that all three structures were involved. Intraoperatively, the gallbladder was markedly shrunken and fibrosed, but no stone was evident, and the fistulous communication was narrow, unlike the large erosive fistula into the duodenum that may be associated with gallstone ileus. The common bile duct moreover was normal, with no evidence of Mirizzi syndrome. From the gastric side, the fistula was punched out cleanly with no concerning features. The principal focus on a possible source was the colon, with interesting endoscopic features of macroscopic inflammation starting at the fistulous site and extending ~15 cm distally in the transverse colon. Histological findings however, were inconclusive and not diagnostic of IBD. If not a primary colonic aetiology, another possibility is that gastric acid entering the colon via the fistula caused this non-specific endoscopic and histologic effect.
Fistulae between the stomach and colon, and between the gallbladder and enteric structures, may occur in the context of Crohn’s disease, peptic ulcer disease, malignancy of the stomach, colon or gallbladder, following radiation therapy, medications such as non-steroidal anti-inflammatory drugs, aspirin and steroids, or post-operatively. Patients typically complain of abdominal pain, nausea and vomiting, diarrhoea, halitosis and vomiting, and rarely gastrointestinal bleeding [1]. Diagnosis of gastrocolic fistula can be confirmed with barium enema, which has a specificity of 95%. Endoscopy may miss small, narrow fistulae located in gastric or colonic folds [2]. CT scan with intravenous and oral contrast is also valuable to confirm the diagnosis, delineate the tract, investigate the primary pathology and for preoperative planning [3, 4]. Management should be multidisciplinary and tailored to each individual patient.
There is no previous directly comparable case published to our knowledge. Although ~50% of Crohn’s patients will develop some form of fistula in their lifetime, gastrocolic fistulae are rare. A recent case series reported 28 examples of gastrocolic fistulas secondary to Crohn’s disease, with fistulae taking years or even decades to develop [5]. There have been three cases of complex fistulae involving the gallbladder, stomach and colon reported [6–8], however, these cases originated in the gallbladder in contrast to our case where this is highly improbable. Park et al. reported a 67-year-old female patient who presented with haematemesis, and underwent endoscopy and clipping of a Dieulafoy lesion. On follow-up endoscopy, a cholecystogastric fistula was discovered and later at surgery a complex cholecysto-gastro-colic fistula identified and resected [6]. Hakim et al. describes a complex cholecysto-gastro-colic fistula, which was discovered intraoperatively during hepatic abscess resection from complications of gallbladder disease. The fistula was resected en bloc [7].
In conclusion, in this case, presenting with classical symptoms and signs of gastro-colic fistula, but where investigations revealed a fistula into her gallbladder in addition, extensive work up revealed no clear aetiology. Notwithstanding, surgery was successful and her clinical outcome excellent. Follow up will include continued gastrointestinal surveillance for IBD.
PATIENT CONSENT
Informed patient consent was obtained for the publication of case details and associated imaging.
AUTHOR CONTRIBUTIONS
Dr Shane Irwin: Lead author, article conception and initial drafting. Final approval and agreement to be accountable for all aspects of the work. Mr Noel Edward Donlon: Contribution to article content, critical draft revision, final approval and agreement to be accountable for all aspects of the work. Ms Helen Mohan: Contribution to article conception, critical draft revision, final approval and agreement to be accountable for all aspects of the work. Prof. John V. Reynolds: Contribution to article conception, critical draft revision, final approval and agreement to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
None declared.
FUNDING
No funding was received by the authors of this case report.
Dr Shane Irwin: Lead author, article conception and initial drafting. Final approval and agreement to be accountable for all aspects of the work. Mr Noel Edward Donlon: Contribution to article content, critical draft revision, final approval and agreement to be accountable for all aspects of the work. Ms Helen Mohan: Contribution to article conception, critical draft revision, final approval and agreement to be accountable for all aspects of the work. Prof. John V. Reynolds: Contribution to article conception, critical draft revision, final approval and agreement to be accountable for all aspects of the work.



