-
PDF
- Split View
-
Views
-
Cite
Cite
Ryusuke Saito, Naoki Tanaka, Takashi Aizawa, Hirofumi Imoto, Akihiro Yamamura, Takeshi Aoki, Naoki Kawamorita, Hiroaki Musha, Shinobu Ohnuma, Fuyuhiko Motoi, Akihiro Ito, Takashi Kamei, Takeshi Naitoh, Michiaki Unno, Tips for operation of inguinal hernia after implantation of artificial urinary sphincter following radical prostatectomy: report of two cases, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa150, https://doi.org/10.1093/jscr/rjaa150
Close - Share Icon Share
Abstract
Urinary incontinence is one of the common complications after radical prostatectomy along with inguinal hernia. Artificial urethral sphincter implantation is widely accepted as a treatment option. We report two surgical cases of inguinal hernia after artificial urethral sphincter implantation for urinary incontinence following radical prostatectomy. In Case 1, since the device went through the inguinal canal, adhesion around the pubis was extremely hard. In Case 2, the device was placed on the ventral side of the rectus abdominis muscle, so it was operable almost as normal. In each case, the surgical procedure was considered carefully after confirming the location of the device by preoperative computed tomography and ultrasonography. Hernia repair was successfully performed using the Lichtenstein method. There are few reports regarding surgical repair of inguinal hernia following artificial urinary sphincter implantation. Preoperative image and appropriate choice of approach could facilitate safe and secure surgery.
INTRODUCTION
Radical prostatectomy (RP) for clinically localized prostate cancer is a widely accepted treatment [1]. There are some distressful complications after RP, which influence the quality of patients’ life such as urinary incontinence, impotence and inguinal hernia (IH). The incidence of IH after open RP and laparoscopic RP is reported to 24 and 14%, respectively, which is extremely higher than that in natural populations [2, 3]. There are some options for hernioplasty after RP and lower abdominal surgery, including transabdominal preperitoneal approach, totally extraperitoneal approach and anterior approach [4, 5].
For the treatment of urinary incontinence, an artificial urinary sphincter (AUS) was introduced in 1972 and it is still the gold standard for the management of incontinence with excellent outcomes [6]. The AUS consists of three parts including an inflatable cuff, a pressure regulating balloon and the control pump [6]. With squeezing of the pump in the scrotum, fluid flows from cuff located around the urethra to the pump and then the cuff is kept in the open position (Fig. 1).
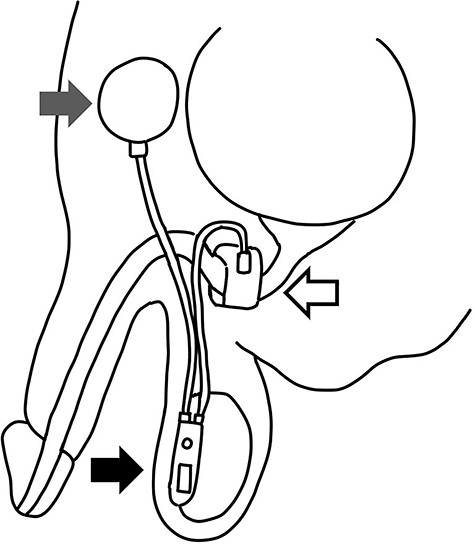
Schema of AUS: the AUS consists of three parts including an inflatable cuff (white arrow), a pressure regulating balloon (gray arrow) and the control pump with tube (black arrow).
Here, we report two cases of IH after AUS implantation following RP. We present two cases of indirect hernia and tips for operation in this report.
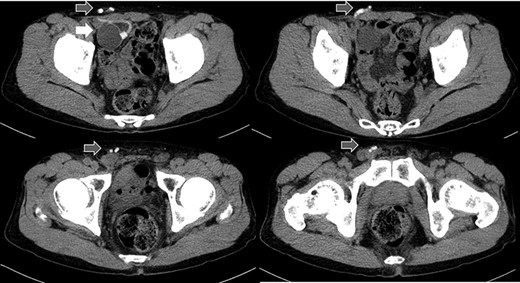
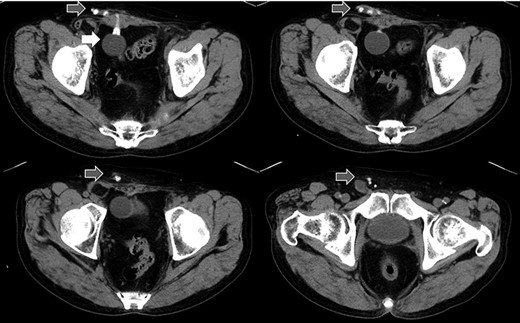
Abdominal CT in Case 1: CT showed that there was a balloon nearby the bladder and the tube went beside the rectus abdominis muscle from abdominal cavity; balloon, white arrow; tubes, gray arrows.
CASE REPORT
Case 1
A 79-year-old male was referred to our institute for the treatment of right IH. He had received AUS implantation for the treatment of incontinence after RP for prostate cancer. Abdominal
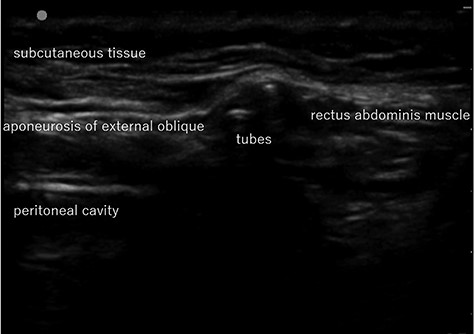
Abdominal US image in Case 1: US showed the tubes running under the aponeurosis of the external oblique that is the anterior wall of the inguinal canal.
computed tomography (CT) showed right IH and a balloon that was placed by the bladder and a connecting catheter that went through the lateral side of the rectus abdominis muscle (Fig. 2). In the operation, we performed ultrasonography (US) at first and it demonstrated that the catheter was running through the inguinal canal (Fig. 3). When we incised the aponeurosis of the external abdominal oblique muscle, the catheter of the AUS was observed in the inguinal canal (Fig. 4). We identified the hernia sac protruding from the inguinal ring and diagnosed it as an indirect IH. The adhesion was too hard to separate the catheter from the pubis and posterior wall of the inguinal canal around pubis. Then, the IH was repaired using the Lichtenstein technique; however, the mesh near the pubis could not be spread as ordinarily.
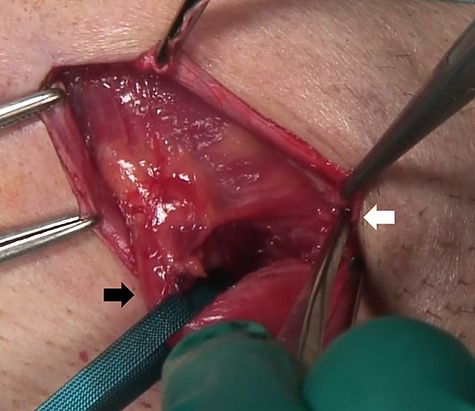
At the operation, the catheter of the AUS was observed in the inguinal canal; tubes, white arrow; spermatic cord, black arrow.
Case 2
A 75-year-old male was referred for the treatment of a right IH. He also had received AUS implantation for a similar medical course. Abdominal CT demonstrated that a balloon was located beside the bladder and that the connecting catheter went through the rectus abdominis muscle and reached to the right scrotum through the subcutaneous layer (Fig. 5). The catheter was palpable subcutaneously; therefore, skin incision was placed on the outer site rather than in the normal situation. The diagnosis was indirect hernia and we did not observe any components of the AUS in the inguinal canal and repaired it using the Lichtenstein method.
Abdominal CT in Case 2: the balloon was located near the bladder and the tube went through rectus abdominis muscle; balloon, white arrow; tubes, gray arrows.
DISCUSSION
IH is one of the common complications after RP and surgical repair should be conducted in order to relieve the symptoms and prevent from incarceration. On the other hands, urinary incontinence is also major complication after RP and the implantation of AUS would be a standard treatment [6]. When IH occurred after AUS implantation, we should pay special attention for management of the hernioplasty in order not to damage the devices. In this report, we experienced two cases of IH after AUS implantation.
Although the spontaneous incidence rate of IH in adult male is estimated to be 5% [7], IH occurs in 24% of patients after open RP and 14% after laparoscopic RP at most [2, 3]. Most types of IH after RP are indirect and the incidence rate is reported to reach up to 91–100% [7, 8]. The reason is uncertain, but there is a possibility that abdominal wall damage associated with the surgery weakens the internal inguinal ring, potentially causing an open peritoneal sheath that develops indirect IH [9]. In addition, postoperative adhesion of the posterior wall of the inguinal canal could prevent direct IH. The two cases we experienced were both indirect types. In general, increased abdominal pressure during urination is one of the major factors related to IH [10]. Although the urinary straining of patients was low before AUS implantation due to severe urinary incontinence, it remarkably increased after AUS implantation. That could be an important factor for IH development in our cases.
There is a report that contraindicates the laparoscopic repair for IH because of scaring and adhesion after RP in the preperitoneal space [4]. In addition, it is difficult to avoid damage to the AUS device without tactile perception. Therefore, we chose an anterior approach, which is the most familiar method for us. The operation of Case 1 patient was difficult because of adhesion in the inguinal canal, but Case 2 was not. The difference between the two cases was whether the catheter went through inguinal canal or not. We could detect the tract of the catheter by preoperative CT and US, which could be a good predictor for adhesion or difficulty in the operation.
In conclusion, although IH after AUS implantation is rare, as the implantation of AUS increases, such cases will increase because of the high incidence of IH after RP. Preoperative management including CT, knowledge of the AUS devices and proper choice of procedure for hernia repair are necessary for safe and secure surgery.
ACKNOWLEDGMENTS
We would like to thank the Tohoku University Center of General Surgery and Urology for the use of its facilities.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
REFERENCES
- ultrasonography
- computed tomography
- adhesions
- hernia, inguinal
- inguinal canal
- preoperative care
- pubis
- rectus abdominis
- surgical procedures, operative
- urinary incontinence
- urinary sphincter, artificial
- urethra
- radical prostatectomy
- inguinal hernia repair
- hernia repair
- medical devices
- stress incontinence after prostatectomy
- sphincter



