-
PDF
- Split View
-
Views
-
Cite
Cite
Aakriti Yadav, Uttam Krishna Shrestha, Kajan Raj Shrestha, Dinesh Gurung, Thoracic aortic aneurysm causing aorto-esophageal fistula—our experience with a rare disease, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa242, https://doi.org/10.1093/jscr/rjaa242
Close - Share Icon Share
Abstract
Aorto-esophageal fistula is a life-threatening condition, accounting for a small number of cases of upper gastrointestinal bleeding where patients present with one or more features of Chiari’s triad. We present the case of a 43-year-old woman, referred to us with symptoms of central chest pain, sudden onset dysphagia followed by massive hemoptysis. She was diagnosed with an aorto-esophageal fistula due to a ruptured thoracic aortic aneurysm and rushed for an emergency endovascular thoracic aortic stent and feeding jejunostomy with intravenous antibiotics and supportive care. After 6 weeks of surgery, the patient was re-evaluated to plan for an esophageal stent if required. The purpose of this presentation is to make the surgical fraternity aware of the gravity of this disease and novel techniques to manage it.
INTRODUCTION
Thoracic aortic aneurysms account for about two-thirds of all aorto-esophageal fistulas (AEFs). Early and aggressive treatment is required, as there is risk of massive bleeding, infection, sepsis and ultimately death. Conservative medical management results in no late survival [1].
Several types of treatment have been described, including open surgery, temporary control measures such as percutaneous embolization and the use of a Sengstaken–Blakemore tube and, more recently, endovascular repair. Infected AEF with poor clinical condition renders them at high risk for open surgery. Endovascular surgery provides palliative treatment or a temporary alternative until patients are healthy enough to tolerate open surgery.
We present a case report of a 43-year-old woman who presented at our institute with a thoracic AEF and underwent thoracic endovascular aortic repair (TEVAR) as a life-saving procedure.
CASE REPORT
A 43-year old women was rushed to the nearest hospital with presenting complaint of mild epigastric pain since two days followed by sudden onset moderate to severe central chest pain, dysphagia and progressive difficulty in breathing. She then developed two episodes of hematemesis with melena. She is an active smoker with no known co-morbidities. The patient was then rapidly resuscitated with multiple blood transfusions and supportive care to stabilize her vital parameters and referred to our center for further management.
On presentation to our center, the patient was pale looking with stable vitals. Chest and abdomen examination was unremarkable. Coagulation profile and cardiac enzymes were within normal limits. However, there was a fall in serial hemoglobin.
Ultrasound of abdomen and pelvis was normal. Upper gastrointestinal tract (UGI) endoscopy was done immediately, which revealed ulceration with a blood clot at 25 cm of the esophagus (Fig. 1).
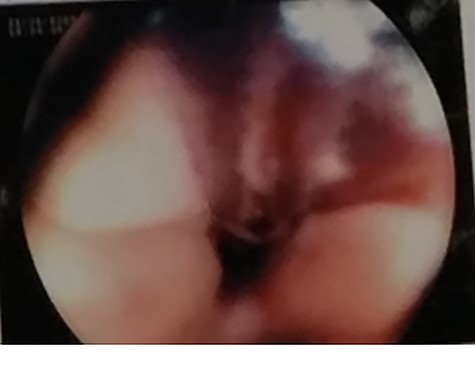
UGI endoscopy, clot with underlying ulceration in the esophagus.
Repeat chest X-ray showed developing left-sided pleural effusion.
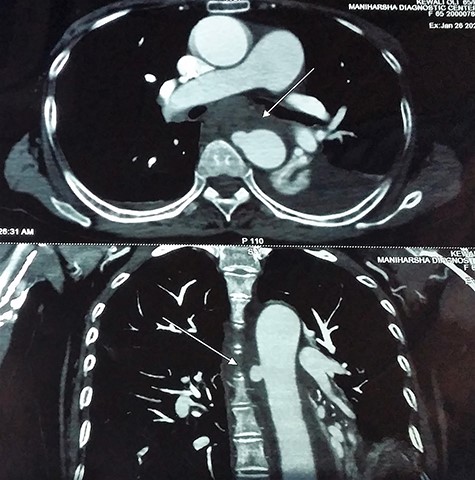
Contrast-enhanced chest computed tomography (CE-CT) with thoracic and abdominal aortogram showed a saccular aneurysm arising from the right lateral wall of the proximal descending thoracic aorta (10 × 8 mm, neck 8 mm) with peri-aneurysmal hematoma tracking along the left posterior mediastinum, causing anterior displacement of the esophagus and a left-sided hemothorax suggestive of a ruptured aneurysm (Figs 2 and 3).
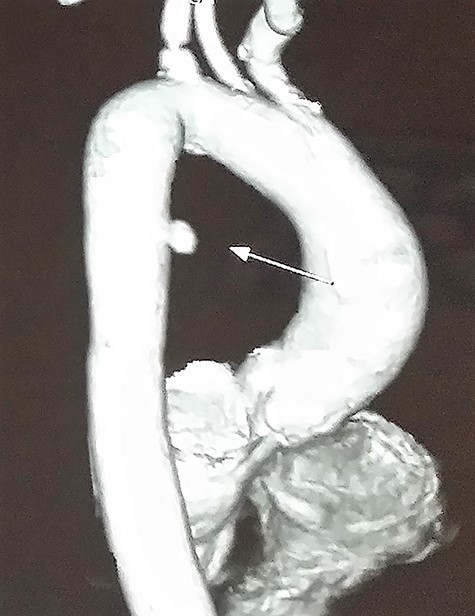
CE-CT image showing saccular aneurysm arising from the proximal descending thoracic aorta.
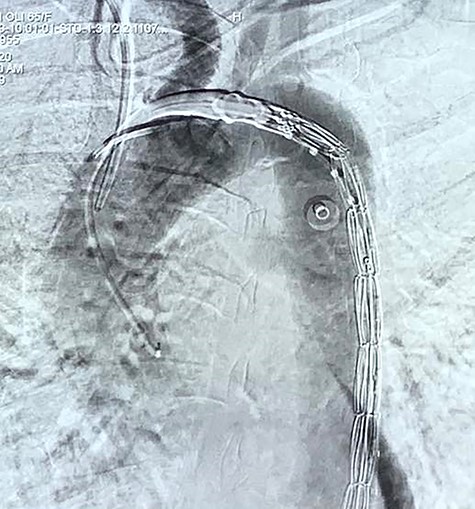
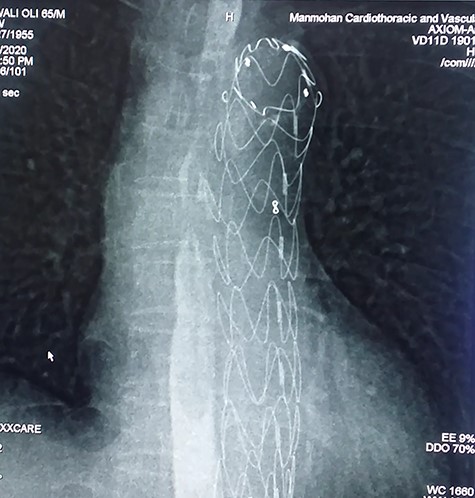
With the above findings, a diagnosis of ruptured descending thoracic aortic aneurysm with AEF was made. The patient was immediately taken for emergency TEVAR where 34 × 34 × 167-mm stent graft (Medtronic Inc. Valiant Thoracic Stent Graft) was deployed using a guidewire beyond the left subclavian artery till above the diaphragm via an incision over the left common femoral artery. Check angiogram showed no leak (Figs 4 and 5). Due to existing mediastinitis, feeding jejunostomy (FJ) was preferred over esophageal stenting. FJ was performed in the same setting after the endovascular procedure.

Post-operatively, she was continued on broad-spectrum antibiotics for mediastinitis with a gradual increase in FJ feed, which she tolerated well. She had an uneventful recovery and was discharged within a week following the procedure.
After 6 weeks, when mediastinitis subsided, gastrograffin swallow was done, which showed no esophageal leak suggesting spontaneous healing of perforation (Fig. 6). Oral feed was commenced with liquid and, subsequently, a normal diet.
DISCUSSION
AEF remains a life-threatening condition with a high rate of morbidity and mortality, described for the first time by Dubreuil in 1818. Incidence of AEF is reported to be 5–23% among all the causes of critical gastrointestinal bleeding by a meta-analysis [2].
Chiari [3] reported the classic clinical triad of mid-thoracic chest pain, sentinel arterial hemorrhage and fatal hemorrhage as ‘aorto-esophageal syndrome’.
Causes of AEF are ruptured or non-ruptured aortic aneurysm, foreign body ingestion and advanced esophageal malignancy. Some cases of AEFs occur as a complication after surgical prosthetic repair of an aneurysm or `following' TEVAR [1].
Snyder and Crawford [4] reported the first survival following surgical repair in 1983, but such cases are few.
The treatment process involves control or prevention of fatal bleeding followed by definitive aortic treatment, accompanied by methods to stop continuous contamination through the fistula to the aortic prosthesis and treating fistulous esophageal communication.
At present, treatment options include either open surgery or endovascular repair. It is advisable that patients with clinical signs of infection and extensive contamination of the surrounding tissues demonstrated by imaging studies, as air bubbles or pleural effusion, or patients with AEF due to foreign body ingestion, should undergo open surgery immediately. The procedure should include debridement of any devitalized tissue, including the compromised segment of the esophagus, and replacement of the diseased aortic segment [5].
In 1994, thoracic endovascular aortic repair (TEVAR) was introduced as an alternative to open surgical repair for treatment of descending thoracic aortic aneurysm and is routinely used in aortic emergencies or high-risk elderly patients. It is the mainstay for providing provisional or palliative treatment, until the patient becomes fit for a definitive procedure. It offers a minimal access procedure saving major operating time and reducing perioperative complications associated with open repair [6].
Recently, there are concerns regarding an increased number of AEF cases after TEVAR. Reports have suggested that TEVAR followed by early open repair (bridging) resulted in better outcomes [7], optimal timing being <1 week; otherwise, the stent-graft is being deeply involved in the infection process. Optimal timing for esophageal reconstruction is at least several months [1].
AEF, being rare with a small number of cases, requires further studies and trials to come up with the best management plan. However, esophageal stenting or reconstruction was not required in our case as esophageal perforation had spontaneously resolved.
CONFLICT OF INTEREST
None.
References
- aortic aneurysm, thoracic
- chest pain
- ruptured thoracic aortic aneurysm
- deglutition disorders
- pathologic fistula
- creation of jejunostomy
- surgical procedures, operative
- upper gastrointestinal bleeding
- esophageal stents
- antibiotic therapy, intravenous
- aortic stents
- massive hemoptysis
- force of gravity
- supportive care
- rare diseases



