-
PDF
- Split View
-
Views
-
Cite
Cite
Rosita Sortino, Michael Schmid, Yassir El Baz, Antonia Loosen, Ignazio Tarantino, Thomas Steffen, Bruno M Schmied, Fariba Abbassi, Indeterminate dendritic cell tumor in the pancreas, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa208, https://doi.org/10.1093/jscr/rjaa208
Close - Share Icon Share
Abstract
Indeterminate dendritic cell tumor (IDCT) is an extremely rare hematologic neoplastic disorder with proliferation of indeterminate dendritic cells. In the vast majority of cases, IDCTs are restricted to the skin or lymph nodes. To our knowledge, we report the first case of IDCT in the pancreas. Due to the rarity of extracutaneous IDCT, guidelines or treatment recommendations addressing their management are missing. We performed a review of literature to compare our experience to the management of other extracutaneous IDCT. Histopathological examination confirms the diagnosis of IDCT in electron microscopy and/or immunohistochemistry. Specific features are the lack of Birbeck granules and the nonreaction to Langerin antibodies. Concerning the aftercare of extracutaneous IDCT, we recommend a dermatological examination to rule out an additional cutaneous manifestation as well as annual blood examinations due to the association between IDCT and hematologic malignancies.
INTRODUCTION
Indeterminate dendritic cell tumor (IDCT) is an extremely rare hematologic neoplastic disorder with proliferation of indeterminate dendritic cells [1]. These cells are considered to be precursors of Langerhans cells sharing some morphological and immunophenotypical features with Langerhans cells. However, the lack of Birbeck granules and the nonreaction to Langerin antibodies in IDCT allow the distinction between these two entities [1].
In the vast majority of cases, IDCT is restricted to the skin or lymph nodes, and there are only rare reports of other locations. To our knowledge, we report the first case of IDCT in the pancreas and compare our experience to the management of other extracutaneous IDCT manifestations described in the literature.
CASE REPORT
A 69-year-old woman was referred to our hospital with acute onset of pain in the upper abdomen and jaundice for 2 weeks.
Relevant laboratory parameters included an elevated serum bilirubin of 360 μmol/l (<20 μmol/l) and a raised Carbohydrate antigen (CA) 19-9 level of 536 kU/I (<35 kU/l), whereas carcinoembryonic antigen (CEA) level was normal.
A computed tomography (CT) scan was performed and showed a poorly defined, hypodense mass in the pancreatic head measuring 2.5 cm and causing obstruction of the common bile duct and pancreatic duct. These findings were consistent with a pancreatic head carcinoma (Fig. 1).
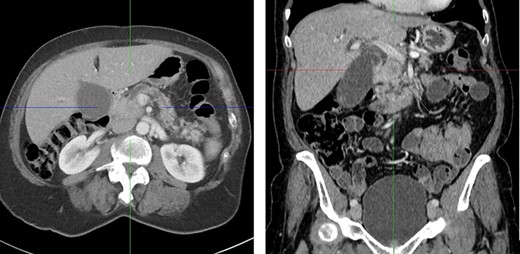
CT scan with a poorly defined 2.5 cm hypodense mass in the pancreatic head causing obstruction of the common bile duct and pancreatic duct. Axial and coronal plane.
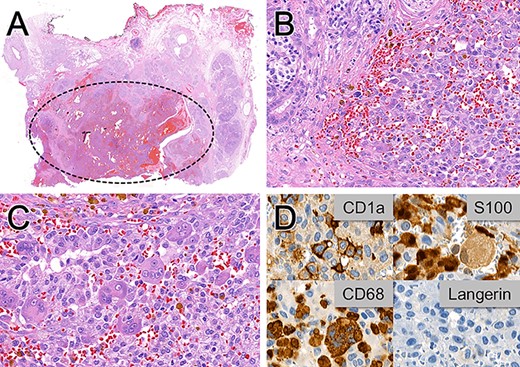
Histopathological (A–C) and immunohistochemical (D) examinations of the pancreaticoduodenectomy specimen. (A) Hemorrhagic, poorly circumscribed proliferation. (B) Medium-sized ovoid cells with variable amounts of granular cytoplasm, indistinct cell boundaries and elongated nuclei with inconspicuous nucleoli infiltrating into the adjacent pancreatic parenchyma. (C) Focal giant cell formation. (D) Variable positivity for the dendritic cell markers CD1a, S100 and CD68 and no immunoreactivity for Langerin. Magnification x6 in A, x300 in B, x400 in C and D.
There were no other suspicious lesions on the thoracoabdominal CT scan. Endoscopic retrograde cholangiopancreatography with brush cytology and placement of a covered metal stent was performed. The cytological findings demonstrated focal mild nuclear atypia and no malignant cells. Due to the imaging characteristics of a malignant lesion and the elevated CA 19-9 level, primary resection was opted. A pylorus-preserving pancreaticoduodenectomy was successfully performed with an uneventful postoperative course.
The histopathological workup of the specimen revealed a hemorrhagic, poorly circumscribed proliferation of medium-sized ovoid cells with variable amounts of granular cytoplasm, indistinct cell boundaries and elongated nuclei, features suggestive of Langerhans cells (Fig. 2).
Immunohistochemistry showed variable positivity for the dendritic markers CD1a, S100 and CD68 but no immunoreactivity for Langerin (CD207) (Fig. 2). These staining features were consistent with a pancreatic IDCT.
DISCUSSION
IDCT is a proliferative hematopoietic disorder and was recently included in the updated WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues [2]. Commonly, IDCT is restricted to the skin or lymph nodes. Other manifestations are extremely rare, with only three cases found in our literature search, including one case in the spleen, one in the spine and one in muscle and parotid [3, 4, 5]. To our knowledge, this is the first reported manifestation of IDCT in the pancreas. Due to the rare occurrence of extracutaneous IDCT, there is no standardized approach for its management.
Extracutaneous IDCT either causes local symptoms due to its size or remains asymptomatic and is an incidental finding. Systemic symptoms have not been described. The lesion is mostly detected by CT scan. Additional investigations, such as biopsies and other imaging modalities, have so far failed to confirm the diagnosis.
To confirm the diagnosis of IDCT, immunohistochemistry and/or electron microscopy are needed. Because indeterminate dendritic cells share many morphological features with Langerhans cells, for example positive immunostaining for CD1a, CD68 and S-100, IDCT might be mistaken for Langerhans cell histiocytosis (LCH). The negative immunostaining for Langerin and the ultrastructural lack of Birbeck granules in IDCT allow the distinction between these two entities. This distinction is essential to avoid an overly aggressive treatment of an IDCT erroneously diagnosed as LCH [1, 6].
Most IDCTs can be cured by surgery [7]. In skin lesions, remission without treatment has even been described. However, associations between IDCT and other hematologic malignancies, such as leukemia and lymphoma, have also been reported [6].
According to the literature, biopsies and cytological examinations of extracutaneous lesions do not lead to the correct diagnosis. Therefore, lesions should be surgically resected, especially if there are malignant features on imaging or elevated tumor markers in blood tests. In our case, imaging characteristics and the elevated level of CA 19-9 were highly suspicious for malignancy and we performed primary resection.
Guidelines for the aftercare of extracutaneous IDCT do not exist. Even if the coexistence of extracutaneous and cutaneous IDCT has not been described in the literature, we think that a dermatological examination should be considered after the diagnosis of extracutaneous IDCT to rule out an additional cutaneous manifestation. In our case, no cutaneous lesion was found. Furthermore, in regard to the association between IDCT and hematologic malignancies, we recommend annual blood examinations.
CONFLICT OF INTEREST STATEMENT
None.
FUNDING
None.
AUTHOR CONTRIBUTIONS TO MANUSCRIPT
Rosita Sortino: concept and design, analysis and interpretation of data, drafting of the manuscript. Michael Schmid: acquisition of histological data, technical and material support, critical revision of the manuscript for important intellectual content. Yassir El Baz: acquisition of radiological data, technical and material support. Antonia Loosen: critical revision of the manuscript for important intellectual content. Ignazio Tarantino: critical revision of the manuscript for important intellectual content. Thomas Steffen: critical revision of the manuscript for important intellectual content. Bruno Schmied: critical revision of the manuscript for important intellectual content, study supervision. Fariba Abbassi: concept and design, analysis and interpretation of data, drafting of the manuscript, study supervision.



