-
PDF
- Split View
-
Views
-
Cite
Cite
Çiğdem Öztürk, Harald J Hoekstra, Patrick H J Hemmer, Jourik A Gietema, Schelto Kruijff, Posterior retroperitoneoscopic resection of recurrent nonseminomatous tumor mass: a case report of the surgical procedure, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa122, https://doi.org/10.1093/jscr/rjaa122
Close - Share Icon Share
Abstract
Treatment of stage II–IV nonseminomatous testicular germ cell tumors (NSTGCTs) consists of cisplatin-based combination chemotherapy and, when present, resection of residual retroperitoneal tumor mass (RRTM) by conventional laparotomy or laparoscopy. In case of a retroperitoneal recurrence, a second conventional or laparoscopic procedure may be challenging.
A case of late relapse after prior conventional resection of a RRTM and tailor-made surgical management with a posterior retroperitoneoscopic resection (PRR) is reported. A posterior retroperitoneoscopic RRTM resection was performed in a 26-year-old male with a history of stage IIC NSTGCT, presenting with a late left-sided retroperitoneal relapse, 6 years after initial treatment. Postoperative course was uneventful and at 1-year follow-up the patient had no evidence of disease. Reoperative surgery by a minimal invasive retroperitoneoscopic approach should be considered as an alternative for patients with a recurrent retroperitoneal tumor mass of a NSTGCT.
INTRODUCTION
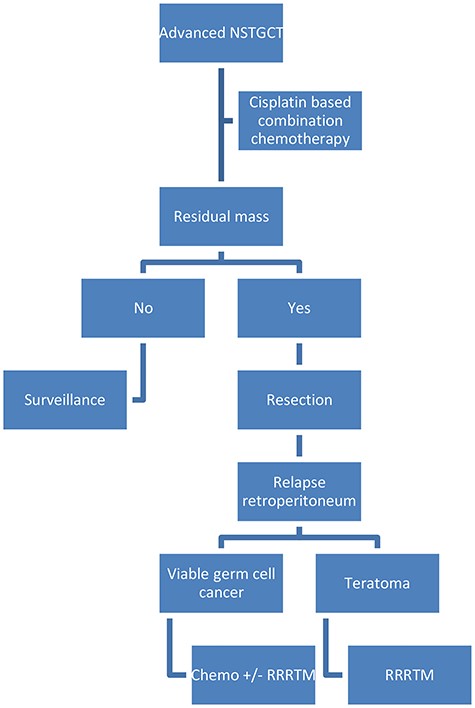
Cisplatin-based combination chemotherapy followed by complete resection of residual masses is the cornerstone of advanced nonseminomatous testicular germ cell tumor (NSTGCT) treatment (Fig. 1) [1, 2]. In 8% of NSTGCT patients treated by chemotherapy and adjunctive surgery, recurrent disease is encountered [3]. In the case of a growing teratoma, resection is performed, whereas in the case of presumed vital tumor, initial treatment will be chemotherapy followed by resection of residual tumor mass if indicated [4].
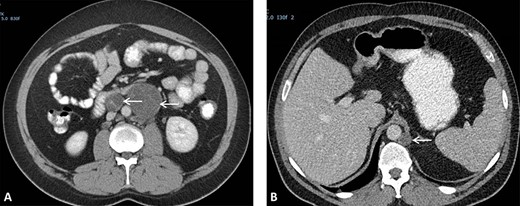
(A) CT scan showing two retroperitoneal masses. (B) CT scan showing retroperitoneal recurrence..
Relapses after prior conventional resections of residual retroperitoneal tumor mass (RRTM) are usually located in the retroperitoneum requiring a laparotomy with extensive surgical exploration. In this case report, an alternative surgical approach, the posterior retroperitoneoscopic resection (PRR), is described.
CASE REPORT
A 26-year-old man was first diagnosed in 2008 with a left-sided testicular tumor treated with inguinal orchiectomy. Resection specimen showed primarily embryonal cell carcinoma and teratoma. The patient, with stage IIC NSTGCT and intermediate-risk group according to International Germ Cell Cancer Collaborative Group [5], received four courses of cisplatin-based chemotherapy. Restaging procedures revealed normalized alpha-fetoprotein (AFP) and a normal beta-human chorionic gonadotropin < 1 U/L. Furthermore, CT scan of the thorax and abdomen showed a left-sided retroperitoneal para-aortic tumor mass, situated caudally of the renal hilus, measuring 6 × 7 cm (prior 5 × 5 cm), and a second retroperitoneal mass situated interaortocaval measuring 1.7 × 1.9 cm (prior 2.6 × 3 cm) (Fig. 2A). Subsequently, the patient underwent a midline laparotomy to resect these tumor masses. Surgical specimens consisted of R0 resection with fibrotic tissue and teratoma with mature and immature compounds. Due to a lack of patient adherence, follow-up protocol could not be executed.
He presented himself 6 years later with a request to resume follow-up, in the absence of symptoms. A retroperitoneal recurrence located at the left retrocrural space with a diameter of 17 mm (Fig. 2B) with normal tumor markers was diagnosed 87 months after initial resection of RRTM. The recurrence, suggestive for a growing teratoma, was located far from the previous operative resection area. The patient was discussed in the multidisciplinary tumor board and offered a posterior retroperitoneoscopic resection of the recurrent disease with the highest chance of a complete resection of this late relapse without surgically dealing with scarred tissues and adhesions caused by a former operation field.
The surgical procedure was performed by two surgical oncologists with experience in performing posterior retroperitoneoscopic adrenalectomy (PRA) [6]. The procedure was performed with the patient in prone position and the surgeon positioned ipsilateral to the tumor and the assistant at the opposite site. The first part of the surgery involved introduction and developing sufficient retroperitoneal space. A first incision was made below the tip of the 12th rib, eventually serving as camera port, and the second port was then placed without camera view on the index finger (Fig. 3). Pneumoperitoneum was created with a high pressure of 25 mm Hg. After having created enough working space, a third 10-mm port was placed. After mobilizing the left kidney from its surroundings and laterally, the tumor mass could be identified. Tumor resection was performed according to the same oncological principles as in conventional resection of RRTM excising only the visible abnormal retroperitoneal tumor mass. Proximal dissection was carefully performed around the left renal artery and vein. The tumor was gently separated off the aorta by blunt and sharp dissection. Finally, the surgical specimen was placed in an endoscopy bag and extracted from the extraperitoneal cavity. The procedure time was 120 minutes. No intraoperative complications occurred. The patient was discharged the next day. Resection specimen showed a R0 resection of a retroperitoneal tumor mass with remnants of mature teratoma. During 12-month follow-up, the patient had no evidence of disease with normal tumor markers and normal imaging (Fig. 4).
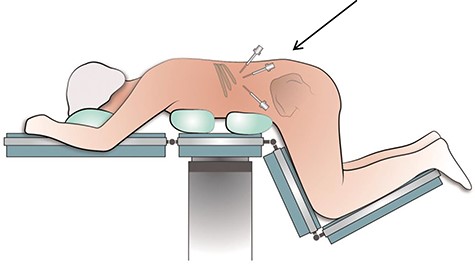
Schematic positioning of a patient in the prone position during the retroperitoneoscopic procedure to excise the RRTM. *Arrow is directed at the port positions; in the middle the camera port is shown.
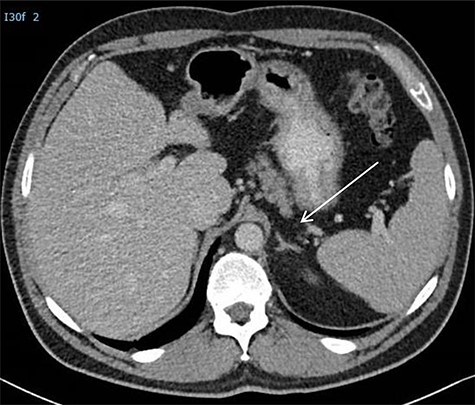
CT scan postoperatively. *Arrow is directed at the adrenal which was not damaged during the procedure.
DISCUSSION
At the UMCG, the current relapse rate in advanced NSTGCT patients treated with cisplatin-based combination chemotherapy and, if indicated, resection residual disease is 18% [7]. Histology shows that teratoma is often present in late relapses and reoperative surgery [7]. Since teratomas are unresponsive to both chemotherapy and radiotherapy, complete resection of all residual tumor masses is an essential part of the combined treatment of NSTGCTs [4]. Since these retroperitoneal relapses tend to be chemoresistant, a selection of patients with anatomically well-defined retroperitoneal disease require reoperative retroperitoneal surgery. Redo surgeries can be technically challenging because of postchemotherapy desmoplastic reaction and annihilated and scarred surgical tissue planes with dense adhesions due to prior surgery [8–10]. Long-term survival varies from 63to 91.3%; factors such as recurrence histology, possibility of salvage chemotherapy, anatomical site of recurrence and experience of the surgical oncologist can explain this wide variance in survival rates [10]. The literature concerning reoperative retroperitoneal surgery in NSTGCTs is limited.
Majority of retroperitoneal recurrences are located in the para-aortic mostly left-sided and interaortocaval regions, making reoperative retroperitoneal surgery challenging [10]. Patients who are candidates for resection of recurrent disease should first undergo accurate staging with CT abdomen and chest and MRI, or even PET-CT might be required. This way, patients with extra-abdominal and non-retroperitoneal disease that cannot be surgically cured are excluded. The surgical goal should always be a R0 resection meaning to resect all abnormal tissues. Pedrosa et al. declared 27% of NSTGCTs patients with a relapse even unresectable after attempting redo surgery [10].
In the current patient, technical challenges were taken into account upfront. Residual tumor mass was situated at a difficult and challenging retrocrural location. With the experience and confidence gained from the PRA, decision was made to perform a PRR instead of a conventional midline laparotomy. Thus, the previous transabdominal surgical route was bypassed, and surgery could be performed partially in a ‘virgin’ territory creating significantly less morbidity.
Today still most of the surgical resections for recurrent NSTGCTs are performed through a conventional midline or transverse exposure. The retroperitoneoscopic technique as used for adrenalectomy might be an alternative option. However, PRR requires a substantial learning time and is technically challenging [6].
CONCLUSION
In reoperative retroperitoneal surgery in NSTGCTs, an alternative surgical strategy such as PRR can avoid the impact of extended conventional surgery or relaparoscopy on the abdominal organs creating less morbidity with respect to bowel and pulmonary functions.
ACKNOWLEDGEMENTS
N/A.
APPROVAL
There is an ethics approval and consent to participate consent for publication of data and material.
COMPETING INTERESTS
All authors have no competing interests.
FUNDING
There is no funding that was used for this report.
AUTHORS’ CONTRIBUTIONS
See specific form also submitted.
CONTRIBUTION AUTHOR(S)
Study concepts: Öztürk C., Hoekstra H.J., Hemmer P.H., Gietema J.A., and Kruijff S. Study design: Öztürk C., Hoekstra H.J., Hemmer P.H., Gietema J.A., and Kruijff S. Data acquisition: does not apply. Quality control of data and algorithms: does not apply. Data analysis and interpretation: does not apply. Statistical analysis: does not apply. Manuscript preparation: Öztürk C., Hoekstra H.J., Hemmer P.H., Gietema J.A., and Kruijff S. Manuscript editing: Öztürk C., Hoekstra H.J., Hemmer P.H., Gietema J.A., and Kruijff S. Manuscript review: Öztürk C., Hoekstra H.J., Hemmer P.H., Gietema J.A., and Kruijff S.
ETHICAL APPROVAL FOR RESEARCH
Yes.
EXTERNAL FUNDING
No.
SOURCE OF FUNDING
Does not apply.
NAME OF PRINCIPAL INVESTIGATOR
Ozturk (If funded, please include a statement as to the role of the study sponsor at end of manuscript under a heading ‘Role of the Funding Source’).
POSSIBLE CONFLICT OF INTEREST
No.
‘All the authors have made a significant contribution to this manuscript, have seen and approved the final manuscript and have agreed to its submission to the BMC Urology’.



