-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Karanikas, Konstantinia Kofina, Dimitrios Potolidis, Soultana Foutzitzi, Savas Deftereos, Eleni Effraemidou, Nikolaos Lyratzopoulos, Spontaneous massive duodenal perforation after ovarian cancer treatment with bevacizumab, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa174, https://doi.org/10.1093/jscr/rjaa174
Close - Share Icon Share
Abstract
Bevacizumab has been used as an effective drug for ovarian cancer. However, serious adverse effects, such as gastrointestinal perforation, can occur. Spontaneous gastrointestinal perforation is an uncommon, yet life-threatening complication related to bevacizumab administration. We present the case of a 65-year-old Caucasian female who presented with acute abdomen 10 days after the first administration of bevacizumab for ovarian cancer treatment, and she was diagnosed intraoperatively with a massive duodenal perforation. Bowel perforation after bevacizumab administration is a serious and potentially lethal complication. Careful follow-up of the patients is necessary in order to detect any signs of this condition in time.
INTRODUCTION
Bevacizumab has been successfully used in cases of platinum-resistant epithelial ovarian cancer, fallopian tube cancer and primary peritoneal cancer, without changes in gastrointestinal complications in heavily pretreated patients [1]. However, bevacizumab has been associated with complications such as bleeding, arterial thromboembolic events and poor wound healing. Gastrointestinal perforation is considered a low-rate complication (2.4%) in any cancer treatment, and it is especially rare in patients with ovarian cancer.
We present a case of massive duodenal perforation 10 days after the first administration of bevacizumab in a female patient treated for ovarian cancer.
CASE REPORT
A 65-year-old woman presented in the emergency department complaining about acute diffuse abdominal pain that had begun 10 h earlier. She did not mention any pre-existing gastrointestinal disorders and was not on regular medication. Her medical history included non-metastasized ovarian cancer, treated with double oophorectomy and salpingectomy through Pfannenstiel incision, 1 month previously. She was given the first dose of bevacizumab as postoperative adjuvant therapy 10 days before the initiation of her present symptoms.
The patient was afebrile but hemodynamically unstable, with 115 bpm and a systolic pressure of 80 mmHg on presentation. Clinical examination revealed abdominal distension, diminished bowel sounds and severe tenderness with peritoneal signs in all of the abdominal quadrants. Her laboratory blood examination showed elevated number of neutrophils (7.77 K/μl), elevated C-reactive protein value (82.46 mg/dl) and decreased Na+ (119 mEq/l). After initial resuscitation, a computed tomography (CT) scan was performed. It showed free abdominal air and fluid in the peritoneal cavity, indicating an intestinal perforation (Figs 1–3).
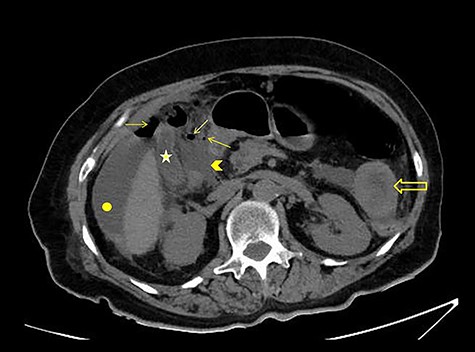
CT scan without intravenous (iv) and oral (per os) contrast media administration: The disruption of lumen continuity at the level of duodenum bulb with presence of fluid (arrow head) and free air (arrows). There are also presence of free fluid in subdiaphragmatic space (dot) and edema in jejunum wall (open arrow). Contracted gallbladder is noted by star.
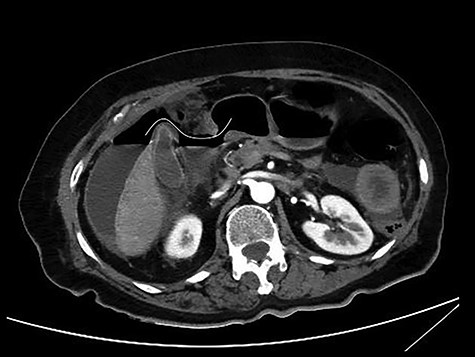
CT scan with iv and without per os contrast media administration: communication of free air with stomach (line).
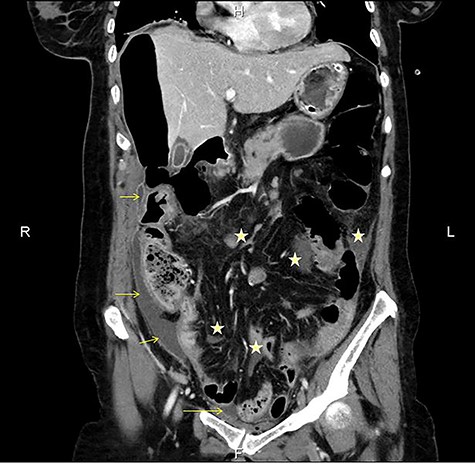
CT multiplanar reconstruction (upper level, supine position) shows free air in continuity with intraluminal (duodenum) air. Notice the presence of free fluid in mesenteric pouches (stars), as well as encapsulated in the right paracolic gutter (arrows) and Douglas space (long arrow).
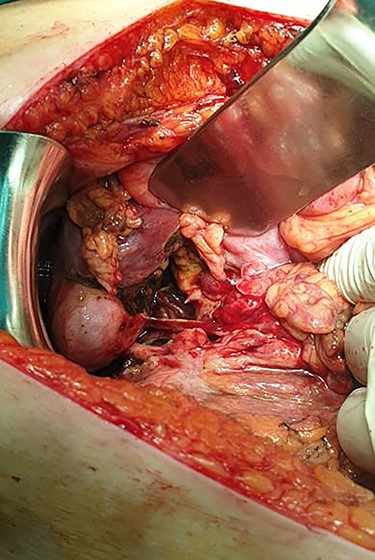
Subsequently, an emergent exploratory laparotomy was decided upon. The exploration of the peritoneal cavity revealed a large amount of dirty fluid, total absence of the anterior and lateral walls of the first part of the duodenum and total bowel discontinuation after the pylorus (Fig. 4). No other disorder or sign of metastasis was present. The pylorus was sutured, a transmesocolic gastroileac anastomosis was performed and a Pezzer tube was placed in the duodenal remnant. Three drains, in Douglas, and in the right and left space were also placed. Empirical triple antibiotic treatment was administered. Postoperatively, the patient remained in the intensive care unit for 24 h. Her hospital stay was further complicated with wound dehiscence and spontaneous low-output enterocutaneous fistula, which were treated conservatively. The patient was discharged in good health on the 28th postoperative day, and the Pezzer tube was safely removed 1 week later.
DISCUSSION
Bevacizumab is a recombinant humanized monoclonal antibody that targets vascular endothelial growth factor and so inhibits the proliferation and maintenance of tumor blood vessels. The drug was enlisted for the treatment of ovarian cancer in Japan in 2013. It is currently used as a first-line treatment, but it is also used in cases of recurrent ovarian cancer [2].
According to a recent review that summarizes the adverse effects of bevacizumab in patients with exclusively gynecologic malignancies, these complications include hypertension, proteinuria, gastrointestinal, respiratory bleeding, thromboembolic events, wound healing impair, gastrointestinal perforation, arthralgia, decreased joint motion and musculoskeletal pain; gastrointestinal perforation is present in only 1.3% of cases. Specifically, bevacizumab-related duodenal perforation is considered extremely rare [3].
Bowel injury, ischemia due to mesenteric vessel thrombosis or vasoconstriction, pre-existing bowel wall invasion from the ovarian tumor and other gastrointestinal disorders, such as obstruction and diverticulitis, have been proposed as possible factors that lead to perforation after treatment with bevacizumab [4]. It has also been suggested that the location of the primary tumor in the abdominal cavity has a key role in the incidence of bowel perforation, especially in cases of colorectal and renal cell cancer. In cases in which intestinal tumors are present, treatment with bevacizumab may lead to tumor necrosis and subsequently, to bowel perforation. However, the exact mechanism underlying gastrointestinal perforation has not yet been fully described.
In our case, the patient presented neither with a metastatic duodenal tumor nor mentioned any pre-existing diagnosis or subjective symptoms of a possible duodenal ulcer prior to bevacizumab therapy; this treatment can thus be suggested to have had a significant effect on intestinal perforation. Therefore, the clinical condition and the intraoperative findings after the first cycle of chemotherapy, in the absence of other risk factors for duodenal perforation, suggest that this perforation was a consequence of bevacizumab administration. Postoperative complications in this patient can also be justified by the adverse effects that bevacizumab induces on surgical wound healing [5].
Chemotherapy-related gastrointestinal complications can occur in any stage of treatment, but most cases of gastrointestinal perforation occur during the early dosing of bevacizumab, as in our case. It is speculated that perforation usually arises in sites of previous resections and anastomoses, patients with bowel resections should be carefully followed up when treated with this drug. Close monitoring of these patients, especially in early stages, ensures the early detection of potentially lethal complications. The re-administration of bevacizumab is not strictly contraindicated after perforation or leakage [6].
In conclusion, bevacizumab is considered a safe therapy in case of ovarian cancer, as serious complications, such as bowel perforation, are rare. However, therapy should be personalized; concerns are raised in patients with pre-existing gastrointestinal conditions, as well as in patients after treatment who present with abdominal pain. Careful patient monitoring may prevent fatalities.
CONFLICT OF INTEREST STATEMENT
None declared.



