-
PDF
- Split View
-
Views
-
Cite
Cite
Karl-Michael Schebesch, Martin Proescholdt, Nils Ole Schmidt, Julius Höhne, Meningioma infiltrating into porous polymethylmethacrylate cranioplasty—report of a unique case, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa149, https://doi.org/10.1093/jscr/rjaa149
Close - Share Icon Share
Abstract
Implantation of a cranioplasty after osteoclastic craniotomy or craniectomy is one of the most common neurosurgical procedures, and polymethylmethacrylate (PMMA) is one of the most frequently applied materials for cranioplasty. This report describes the unique case of a patient with recurrent transitional meningioma WHO I that infiltrated the PMMA cranioplasty 7 years after primary surgery. We propose to restrict the use of porous PMMA as cranioplasty after the removal of convexity meningioma.
INTRODUCTION
Remodeling the calvarial bone with freehand polymethylmethacrylate (PMMA) cranioplasty after craniectomy is a very common neurosurgical procedure. Apart from freehand PMMA application after decompressive hemicraniectomy or postoperative osteomyelitis in stroke patients, some authors have advocated the use of computer-assisted design and computer-assisted manufacturing (CAD/CAM) cranioplasties [1, 2], also after the removal of infiltrated skull or hyperostotic bone in meningioma surgery [3, 4]. PMMA is one of the most frequently applied materials for cranioplasty. To the best of our knowledge, tumorous invasion of PMMA in a patient with recurrent meningioma has never been reported. We describe the case of a patient with recurrent meningioma WHO I that infiltrated the implanted PMMA cranioplasty 7 years after primary surgery.
CASE REPORT
In 2012, the 45-year-old female patient presented at a neurosurgical department with aphasia and facial palsy. Magnetic resonance imaging (MRI) showed a large space-occupying tumor infiltrating the frontal bone. Consequently, the tumor was removed, and histological workup confirmed a benign meningioma WHO I. Two years later, a CAD/CAM non-resorbable biocompatible cranioplasty (BIOMET, Germany) composed of PMMA spherical macro beads, coated and fused with polyhydroxyethylmethacrylate, was implanted, see Fig. 1. Until February 2018, consecutive MRIs had shown a tumor-free area, and the clinical course had been uneventful. The MRI conducted in February 2018 and the subsequent MRI in June 2019 (Fig. 2A and B) showed a progressive contrast-enhancing mass along the falx cerebri that was strongly suspicious of recurrent meningioma. Therefore, revision surgery was recommended to remove the tumorous mass along the falx. Before surgery, computed tomography (CT) was carried out to visualize the bony attachments of the PMMA cranioplasty (Fig. 2C). Neither imaging modality had depicted any tumorous tissue inside the cranioplasty. Thus, preoperatively, the cranioplasty was not considered an area of tumor infiltration.

Model of a CAD/CAM non-resorbable biocompatible cranioplasty (Biomet, Germany) composed of polymethylmethacrylate (PMMA) spherical macro beads coated and fused with polyhydroxyethylmethacrylate (PMHA).
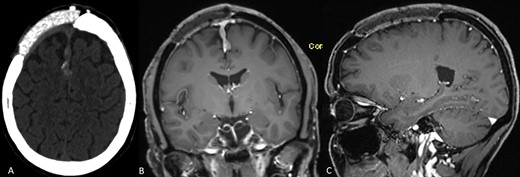
(A–C) Preoperative neuroimaging shows recurrent meningioma along the falx cerebri and under the cranioplasty (A: native CT scan, axial plane; B: contrast-enhanced MRI, coronal plane; C: contrast-enhanced MRI, sagittal plane).
Revision surgery with the intent to remove the recurrent meningioma was conducted in November 2019. The initial PMMA cranioplasty that moderately adhered to the dura was removed in one piece. After splitting of the cranioplasty, several pieces were sent to the department of neuropathology because of the surgeon’s strong impression that tumor tissue had infiltrated the porous material of the cranioplasty. Further surgery was uneventful: the tumor was dissected and completely removed, the convexity dura was reconstructed, and a preformed titanium cranioplasty was inserted. Histologically, the tumor was confirmed as transitional meningioma WHO I.
Immunohistochemical analysis of meningioma cell infiltration
The fragments were frozen in isopentane and embedded in Tissue Tek® OCT compound (Sakura, Staufen, Germany). Samples were cryosected and sections were fixed in 4% paraformaldehyde and washed in phosphate buffered saline. To detect meningioma cell infiltration, cryosections were immunostained using a human epithelial membrane antigen (EMA) specific mouse monoclonal antibody (clone E 29, Agilent DAKO, Santa Clara, CA, USA). To exclude glial cell infiltration, adjacent sections were stained with a polyclonal rabbit anti-human glial fibrillary acidic protein (GFAP) antibody (Agilent DAKO, Santa Clara, CA, USA). To test for unspecific antibody binding, control sections were incubated either in non-immunized mouse IgG (EMA staining) or non-immunized rabbit IgG (GFAP staining) at identical protein concentrations.
Pieces of the infiltrated cranioplasty were microscopically examined after topical staining as described above. Immunohistology clearly identified meningioma cell formations inside the cranioplasty (Fig. 3B and C), and topographical microscopy showed meningioma formations along the preformed caverns (Fig. 3A).
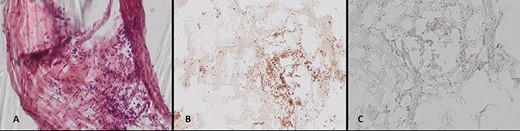
(A–C) Histological work-up of the explanted cranioplasty: (A) hematoxylin/eosin staining, immunohistochemical staining for (B) epithelial membrane antigen, and (C) GFAP (all ×20 magnification).
DISCUSSION
Only a few articles in the neurosurgical literature have focused on tumor infiltration into the cranial flap. In 1994, Wester described six patients undergoing reimplantation of tumor-infiltrated autologous bone flaps after autoclaving. In 1997, Vanaclocha et al. published their experiences in reimplanting autoclaved bone flaps in 62 patients with various benign and malignant skull-infiltrating tumors [5]. In the histological evaluation of bone samples after the autoclaving procedure, the authors found no living tumor cells inside the bone but preserved mineral matrices. This finding, in combination with the low rate of bone resorption during follow-up, resulted in the authors’ conclusion that autoclaved calvarial bone flaps are safe and feasible. It is noteworthy that no long-term follow-up focusing on tumor regrowth or reinfiltration was provided in this large series.
On the other hand, many large studies show a very high rate of up to 25% of surgical revision after reimplantation of autologous bone flaps due to bone resorption [6, 7]. The main reason for replacing bones with artificial materials, such as PMMA, hydroxyapatite bioceramics, titanium and others, is aseptic bone necrosis [8, 9] that can be gradually visualized as a shrinking bone flap in CT. Osteoconductive materials such as hydroxyapatite have been intensively examined histologically. Bruno et al. reported that neo-formed lamellar and trabecular bone tissue fragments that are accompanied by amorphous reticular tissue showed diffuse ossification in explanted bioceramic cranioplasties [10]. This intense osteo-conductive effect obviously facilitates the osseous integration of artificial material into the skull; however, invasive neoplastic engraftment may be potentially devastating, particularly in recurrent skull-infiltrating bone tumors.
Currently, most neurosurgeons prefer the primary removal of the potentially tumor-infiltrated autologous bone flap and replacement with a CAD/CAM cranioplasty [1] or, at least, with a simple PMMA reconstruction.
The most common skull-infiltrating tumor is meningioma, most of which are graded WHO I. To the best of our knowledge, no study has ever evaluated whether recurrent meningioma has the propensity to infiltrate artificial materials covering and integrating into the osteoclastic defect and, if so, which materials have a higher risk of meningioma growth. However, one cannot necessarily assume that tumor growth inside the cranioplasty is significant or results in more extensive tumor regrowth. It is noteworthy that our findings in this unique case are in contrast to the case report of Frassanito and co-workers who presented the case of recurrent atypical meningioma that recurred without infiltrating the hydroxyapatite cranioplasty [4].
However, we report this unique observation that resulted in the departmental decision to stop reconstructing the skull with any porous, bone-like, or osteoconductive material after meningioma surgery. We now exclusively use titanium or a preformed calcium phosphate composition whenever necessary and do not reinsert the autoclaved autologous skull. Further in-vitro and in-vivo studies are mandatory to shed light on the potential of meningioma invasion into cranioplasty.
CONFLICT OF INTEREST STATEMENT
None declared.



