-
PDF
- Split View
-
Views
-
Cite
Cite
Eva María Pueyo-Périz, Patricia Sánchez-Velázquez, Maite De Miguel, Aleksandar Radosevic, Henrik Petrowsky, Fernando Burdío, Replaced right hepatic artery arising from the gastroduodenal artery: a rare and challenging anatomical variant of the Whipple procedure, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa136, https://doi.org/10.1093/jscr/rjaa136
Close - Share Icon Share
Abstract
Accurate assessment of the vascular anatomy is a prerequisite of any pancreatic resection, since an unnoticed arterial injury in the context of a complex resection such as Whipple procedure, can seriously jeopardize patient’s safety. This article aims to describe an infrequent anatomic variant of a replaced right hepatic artery originating directly from the gastroduodenal artery and its potential implications for duodenopancreatectomy, as the gastroduodenal artery is routinely divided. We present here two different cases of this arterial abnormality identified during a Whipple procedure and its implications in each different setting. Preoperative identification of anatomical variations is essential for proficient surgical planning. Nevertheless, when detected during surgery, an meticulous dissection of the hepatoduodenal ligament is required to identify all the vascular relations in order to avoid irreversible damage.
INTRODUCTION
Assessment of preoperative imaging in patients undergoing major pancreatic surgery requires detailed attention in a multidisciplinary setting in order to plan the specific surgical treatment. Any unexpected variation detected during pancreatic surgery requires meticulously vascular exploration to avoid unintentional injury and achieve safe pancreatic head resection with correct oncologic margins.
CASE REPORT
Case 1
A 64-year-old woman was admitted to hospital with painless jaundice later confirmed. Laboratory test revealed elevation of bilirubin (8 mg/dl) and serum tumor marker CA 19-9 and CEA. Further work up by computer tomography (CT) identified a 2 cm hypodense mass within the pancreas head consistent with a pancreatic neoplasm. In absence of vascular involvement and distant metastases, the tumor was assessed as resectable in the multidisciplinary board and the patient was scheduled for surgery. At this point, the preoperative imaging evaluation already identified a replaced right hepatic artery (RHA) arising directly from the gastroduodenal artery (GDA) (Fig. 1).
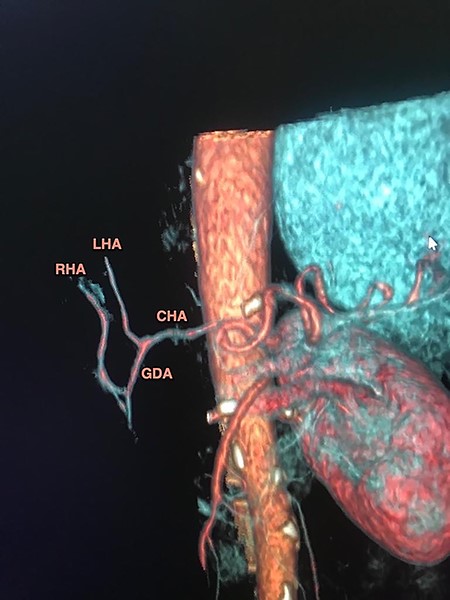
CT-based coronal 3D reconstruction shows the GDA runs down towards the pancreatic mass and the RHA arises from de GDA before entering the pancreas head.
This variant was intraoperatively verified. When the proper hepatic artery (PHA) was dissected out, it became evident that the PHA merged in a single left hepatic artery (LHA) supplying exclusively the left lobe. Further dissection toward the pancreatic mass revealed that the RHA had its origin 1 cm below the bifurcation of the GDA from the common hepatic artery (CHA) (Fig. 2A). However, the LHA and PHA were identified as expected. The ‘real’ GDA was assumed to be encased by the tumor, as the distal aspect of the artery was extremely close to the upper border of the mass. After careful dissection, the GDA was distally divided from the RHA (Fig. 2A) and the mass was removed with correct oncologic margins, following a classical duodenopancreatectomy and Child reconstruction. Figure 2B shows the replaced RHA after removal of the specimen and division of the GDA. The postoperative course was uneventful and was discharged on the postoperative day 11.
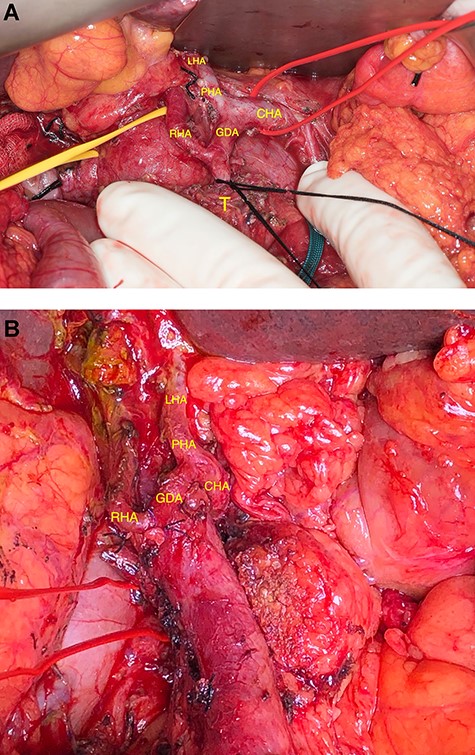
(A) Arterial anatomy before resection. (B) Arterial anatomy after resection.
Case 2
A 78-year-old otherwise healthy female patient was diagnosed with pancreas adenocarcinoma following a biliary sepsis in November 2017. Abdominal CT scan identified a mass within the head of the pancreas without vascular invasion and absence of distant metastasis. Previous to surgery, endoscopic retrograde cholangiopancreatography was performed with bile duct drainage, and fine needle aspiration confirming the diagnosis of adenocarcinoma. The case underwent multidisciplinary tumor board discussion and was deemed as resectable according to the American Joint Committee on Cancer TNM staging system and scheduled for surgery. Already at the time of preoperative staging, an aberrant right hepatic artery (aRHA) arising from the superior mesenteric artery (SMA) had been acknowledged.
Based on the preoperative finding, SMA was first approached during surgery [1] in order to identify the origin of the preoperatively recognized aRHA. After identifying the RHA in the posterolateral aspect of the common bile duct (CBD) (Fig. 3), it was followed proximally to its origin from the SMA to ensure safe resection. According to the previous case, an additional replaced RHA originating from the GDA was intraoperatively identified. Then, the GDA was temporarily occluded with a Bulldog clamp in order to verify the flow through the RHA, which proved to be sufficient by Doppler. Despite both challenging arterial variants, the pancreatic mass was removed with preservation of both the aRHA originating from the SMA and the replaced RHA originating from the GDA following the division of the GDA close to the origin of the replaced RHA. Duodenopancreactectomy was completed correctly and achieved tumor-free margins. No postoperative complications occurred and the patient was discharged on postoperative day 15.
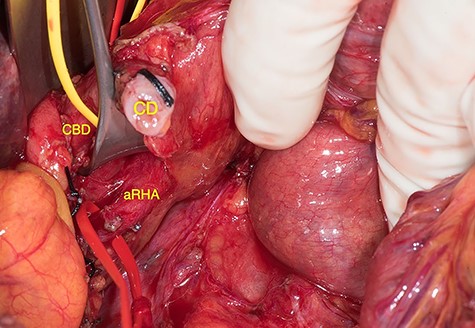
aRHA below the cystic duct (CD), encircled by the vessel loop, arising from the SMA; RHA was identified in the posterolateral aspect of the CBD.
DISCUSSION
Surgical resection currently remains the only potentially curative treatment for pancreatic cancer, while Whipple procedure is the standard option in many institutions [2]. Despite the technical advancements, duodenopancreatectomy remains a complex operation with significant morbidity and mortality rates, ranging from 1 to 2% even at experienced centers [3]. To make things even more challenging, the celiac trunk anatomy is hampered by high variability, some of which are extremely rare inadvertent variations that might not be recognized during the preoperative study [4]. Classical anatomical variants of hepatic arterial vascularization have been widely described in Michels’s classification and categorized into 10 basic types [5, 6]. Focusing specifically on RHA, it originates from the PHA in ~80% of cases and is considered as the standard anatomy. There is a certain confusion in the literature when to use exactly the terms ‘aberrant’, ‘replaced’ and ‘accessory’. In 15% of cases, the RHA can be replaced (i.e. following a path different to the standard course) most often arising from the SMA (Michel’s Type III). In 5%, is an accessory RHA (i.e. an extra blood supply, apart from the main artery) arising from the SMA, celiac trunk or aorta [7–9]. Both described conditions are considered as aberrant artery.
However, there are variations that are not included in this classification. In fact, this case report describes two scenarios with anatomic variations of the RHA originating directly from the GDA in the setting of a resectable adenocarcinoma of the pancreatic head. During pancreaticoduodenectomy, the GDA is usually divided at its origin from the PHA. However, when the RHA originates from the GDA, surgical dissection can be extremely dangerous and might misdirect the surgeon to divide the RHA instead of the GDA at its usual location. Thus, awareness of not only standard vascular anatomy but also of rare but problematic anatomical variants is of the paramount importance to avoid iatrogenic injury of the right hepatic lobe blood supply while performing safe resection. Therefore, meticulous dissection of the arterial vessels within the hepatoduodenal ligament is mandatory for complete arterial identification before any critical structure might be divided and cause arterial damage.
Both cases highlight the importance of preoperative radiological assessment especially CT-based 3D reconstruction since early detection of rare arterial variants offers assistance in preoperative planning and modifies the surgical approach However, rare arterial variations are sometimes missed by preoperative imaging and are surprisingly detected during pancreatoduodenectomy. In this scenario, the surgical strategy needs to be adjusted during surgery in order to achieve adequate resection margins and avoid potential pitfalls.
CONFLICT OF INTEREST STATEMENT
All authors reported no competing interest.
FUNDING
Funding requirements: Instituto de Salud Carlos III-FEDER (PI17/00468).



