-
PDF
- Split View
-
Views
-
Cite
Cite
Agdaliya Mikhalkova, Markus Hoffermann, Extensive subdural spread of a glioblastoma associated with subdural hygroma: case report, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa127, https://doi.org/10.1093/jscr/rjaa127
Close - Share Icon Share
Abstract
Despite its highly malignant behaviour, glioblastoma very rarely spread beside the arachnoid layer. We describe a very rare case of a 67-year-old patient with glioblastoma, who developed a recurrent subdural hygroma associated with the subdural spread of the glioblastoma, which was confirmed histologically. Possible predisposing factors and management suggestions are discussed.
Introduction
Glioblastoma represents one of the most common intracranial tumours in adults. With improved imaging and survival, leptomeningeal glioblastoma metastases are reported with growing frequency [1]. However, metastatic spread beyond the subarachnoid space or outside the central nervous system remains seldom. We describe a very rare case of the subdural glioblastoma spread associated with a hygroma.
Case report
A 67-year-old male patient presented at a local hospital with progressive fatigue, memory and concentration loss, right-sided neglect and weakness as well as gait disturbance. A cerebral MRI scan depicted an inhomogeneous contrast-enhancing, left temporo–parieto–occipital intracerebral tumour invading the trigonum of the left lateral ventricle. The tumour was resected subtotally under 5-ALA fluorescence with a small remnant infiltrating the trigonum of the left lateral ventricle, which was opened intraoperatively (Fig. 1). Histologically, glioblastoma multiforme, WHO IV°, was diagnosed. No molecular analysis was performed.
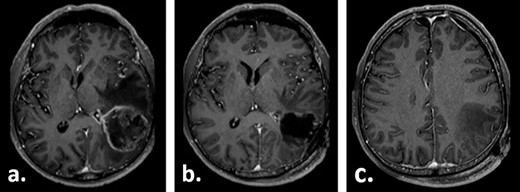
Gadolinium-enhanced T1-weighted axial MRI of the patient preoperatively (a) and 1 day after the tumour resection (b, c).
Postoperatively, the patient received fractionated radiotherapy of the tumour region up to 62 Gy and concomitant chemotherapy with temozolomide 75 mg/m2 body surface area for 44 days, starting in the third postoperative week.
Ten weeks postoperatively, the patient complained of vertigo and a self-limiting episode of right-sided visual field restriction and neglect. Subsequently, oral cortisone therapy was started again, which lead to the relief of these symptoms. The MRI showed a new contrast enhancement around the resection cavity, possibly treatment-related after radiochemotherapy, as well as a 14-mm-thick, left-sided subdural fluid collection causing a midline shift of 6 mm, which was interpreted as a subdural hygroma or hematoma (Fig. 2). The latter was slightly progressive on a CT scan 1 week later, and surgical evacuation through a burr hole was indicated. Intraoperatively, it presented as a slightly xanthochrome, slightly pressurized subdural hygroma. No pathological alterations of the dura were revealed during this procedure. Postoperatively, the subdural hygroma was incompletely regressive and began to grow again as was seen on the CT images 3 weeks after the surgery. This finding was considered to be related to post-radiation changes. The patient stayed asymptomatic and received two cycles of adjuvant chemotherapy with temozolomide 150 mg/m2 body surface area and 200 mg/m2 body surface, respectively.
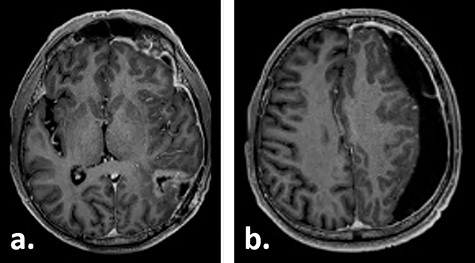
Gadolinium-enhanced T1-weighted axial MRI of the patient 10 weeks after tumour resection demonstrating the resection cavity (a) and new subdural fluid collection (b).
Fifteen weeks after tumour resection and 4 weeks after hygroma evacuation, the patient presented again with concentration loss, fatigue and a moderate right-sided hemiparesis. A new CT scan revealed a strongly progressive subdural hygroma with a 3-mm-thick subdural membrane. The hygroma was evacuated through the same burr hole; however, due to an unsatisfying hygroma regression, a subduro–peritoneal shunt for permanent hygroma drainage was implanted subsequently. During these both procedures, a thick greyish subdural membrane was noticed, but no tissue samples were taken. Despite the volume reduction of the hygroma, its membrane still caused a considerable mass effect. Few days after the last surgical procedure, a cerebral MRI was performed following the tumour follow-up protocol. On this imaging, the subdural membrane over the left hemisphere presented as an inhomogeneous contrast-enhancing lesion with irregular contours (Fig. 3).
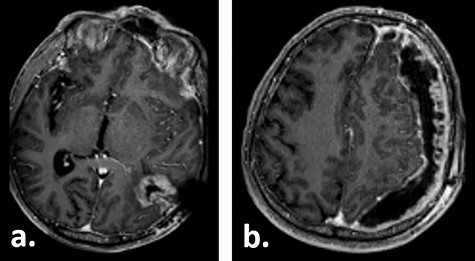
Gadolinium-enhanced T1-weighted axial MRI of the patient 16 weeks after tumour resection demonstrating the resection cavity (a) and progressive subdural fluid collection with the newly occurred contrast-enhancing membrane (b).
Subdural spread of the glioblastoma was suspected, which was validated by an open tissue biopsy. Intraoperatively, the membrane appeared as a beige, thick subdural mass with a xanthochrome hygroma underneath. Histologically, a subdural metastasis of the underlying glioblastoma was confirmed. The molecular status of the tumour could not be determined due to DNA degradation in the tissue sample. Our reference pathology centre considered gliosarcoma as a differential diagnosis.
Due to the substantial tumour progress under chemotherapy and dramatic worsening of the patient’s condition, no further surgery or chemotherapy was performed.
The patient developed signs of increased intracranial pressure and died 5 months after the initial diagnosis of glioblastoma.
Discussion
Several cases of different intracranial tumours mimicking subdural hematoma, mostly adenocarcinoma metastases, have been described in the literature [2]. However, despite its aggressive growth, glioblastomas rarely metastasize beyond the subarachnoid space. Malignant gliomas usually spread along pre-existing vessels and additionally stimulate neoangiogenesis. They expand along white matter tracts through tissue destruction and growth, while the dura mater usually poses a robust barrier for further spread.
However, a local postoperative and even spontaneous transdural extracranial as well as leptomeningeal and intramedullar glioblastoma spread can rarely occur. Recraniotomy, an aggressive glioma type, as well as chemo- and radiotherapy, may also increase the risk of tumour dissemination inside the central nervous system. Additionally, intraoperative entering into the ventricles was described as a risk factor for leptomeningeal spread. Furthermore, peritoneal and systemic metastases were described in patients with glioblastoma and ventriculoperitoneal shunt [1, 3]. However, in a recent meta-analysis of extracranial and extraspinal glioblastoma metastasis, 88.7% of the patients did not have a ventriculoperitoneal shunt [4].
Due to the initial absence of pathological contrast enhancement of the hygroma membranes, we believe that the subsequent surgeries might have caused the subdural tumour dissemination. Lettau et al., who developed a subdural hygroma followed by multiple supra- and infratentorial dural metastases including an extensive fronto–parietal subdural mass, presented a similar case report of a patient with temporal glioblastoma multiforme. The authors presume the placement of a subdural catheter could have led to penetration of the arachnoidal layer and consecutive subdural tumour spread [5]. In our patient, the subdural catheter was placed frontally, remotely from the occipitally located primary tumour. However, intraoperative subdural irrigation could have led to the additional spread and dural implantation of the tumour cells already present in the CSF.
Gliosarcoma may arise secondarily from a glioblastoma. Some of these tumours resemble meningiomas and have a rather higher tendency to metastasize than glioblastoma [6]. In accordance with that, the suspected secondary transformation into gliosarcoma could have additionally promoted the extensive tumour spread in our patient.
Retrospectively, the management of the case could have been improved, if histological samples were taken at the time of the second surgical intervention for the recurrent subdural hygroma. At this point, underlying tumour dissemination could have been suspected.
In conclusion, very rarely glioblastoma may develop an extensive subdural spread, which might be facilitated by the placement of a subdural catheter or a secondary transformation into gliosarcoma.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to Ms Jennine Vari for her proofreading of the article.
Conflict of interest statement
None declared.



