-
PDF
- Split View
-
Views
-
Cite
Cite
Abdul Rauf, Stephanie F Smith, Rono Mukherjee, Nyla Nasir, Not such a small diagnosis: small cell carcinoma of the prostate, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa117, https://doi.org/10.1093/jscr/rjaa117
Close - Share Icon Share
Abstract
Small cell carcinoma (SCC) is an aggressive malignancy most commonly described in the lung. We present a case of a 61-year-old male who presented with a neck swelling and was subsequently found to have metastatic SCC of the prostate. Clinicians should be aware that it metastasizes early. Unlike conventional prostate adenocarcinoma, it is not a prostate-specific antigen (PSA) secreting tumor hence serum levels do not correlate with disease severity, and a low PSA reading may give false reassurance. In the future, further studies on genomic typing and novel targeted therapies may achieve better clinical outcomes for patients with this aggressive type of prostate cancer.
INTRODUCTION
Small cell carcinoma (SCC) is an aggressive malignancy most commonly described in the lung. It is estimated that ~4% of SCC cases are extrapulmonary (EPSCC) [1]. In the UK, prostate cancer is the most common cancer in men, with an estimated 48 500 new prostate cancer cases yearly [2].
Small cell carcinoma of prostate (SCCP) accounts for just 0.5–2% of all cases of prostate cancer [3]. It is a high-grade malignant neoplasm with neuroendocrine differentiation. It is distinct from conventional prostatic adenocarcinoma in its biological behavior and clinical presentation, which necessitates a different treatment approach [4, 5]. We present a case report of a patient diagnosed with SCCP presenting as a neck lump, highlighting its aggressive nature and diagnostic challenges.
CASE REPORT
A 61-year-old male presented with a left-sided neck swelling. He underwent fine-needle aspiration (FNA) for investigation of suspected lymphoma. This revealed metastatic poorly differentiated carcinoma with neuroendocrine features.
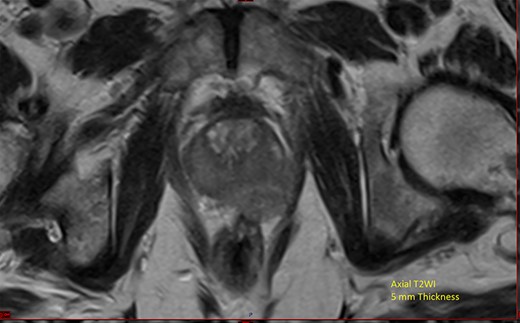
He underwent computerized tomography of the thorax, abdomen and pelvis, which showed lobular enlargement of the prostate with enlarged pelvic, para-aortic and cervical lymph nodes. Prostate-specific antigen (PSA) was elevated at 139 ng/ml. Magnetic resonance imaging (MRI) of the prostate showed extensive local disease (Fig. 1). Nuclear medicine bone scan revealed multiple sclerotic bony metastases.
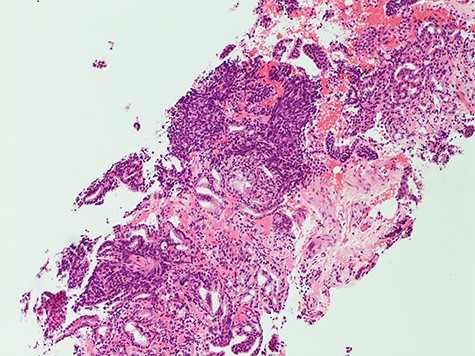
Histology from trans-rectal ultrasound (TRUS) biopsy of the prostate showed a conventional microacinar type prostatic adenocarcinoma of Gleason score 4 + 4 = 8 with an intermingled component of SCC, composed of cohesive sheets of small cells with scant cytoplasm, hyperchromatic nuclei and nuclear molding with increased mitotic activity (Fig. 2). The cytomorphology was similar to the neck FNA. The small cell component showed positive staining for neuroendocrine markers Synaptophysin and CD56. The Ki-67 index was 70%. PSA showed scattered positivity. Overall, the features were consistent with a mixed microacinar adenocarcinoma and SCC. Following the diagnosis from TRUS biopsy, PSA immunostain was carried out on the FNA material, which showed scattered positivity.
Uro-oncology multidisciplinary team discussion recommended oncology referral. He received hormonal therapy in the form of ‘degarelix’ for the prostate adenocarcinoma component and chemotherapy (etoposide and cisplatin) for the SCC component.
DISCUSSION
SCCP is a rare entity. Typically, serum PSA levels are low despite large metastatic burden [4–6]. Approximately half of the cases of SCCP are associated with a conventional acinar adenocarcinoma [4, 5]. SCC commonly emerges in patients with high-grade adenocarcinoma [4].
SCCP is thought to represent trans-differentiation from usual prostate adenocarcinoma [7]. In mixed tumors, the transition between the small cell and acinar components is abrupt on histological examination [7]. As with other unusual subtypes of prostate cancer, a Gleason score is not assigned to SCCP. It is recommended that the percentage and Gleason grade of the acinar component should be provided [7].
The diagnosis of SCCP is based on morphological features defined in the World Health Organization (WHO) classification criteria of pulmonary neoplasms (WHO 2015). From a diagnostic point of view, it is important to recognize this rare variant and not misdiagnose it for a high-grade Gleason 5 pattern, as there is a morphological overlap [5]. In cases where there is morphologic concern for neuroendocrine carcinoma (sheet-like architecture, nuclear molding and scant cytoplasm), it is essential to perform immunohistochemistry with neuroendocrine markers and PSA. In our case, the SCC component was strongly positive for neuroendocrine markers Synaptophysin and CD56 with focal positivity for PSA. In the conventional component it was the reverse; strong PSA positive and very focal NE scattered cells (Figs 3 and 4). This is in line with the literature findings [5, 8].
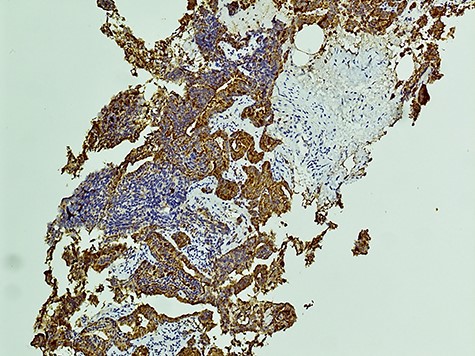
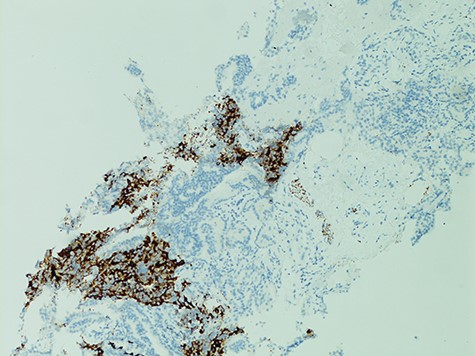
The aggressive nature of SCCP is demonstrated in this case that the patient presented with cervical lymphadenopathy representing nodal metastasis. It has been described that SCCP metastasizes early in its course and brain metastases are common, therefore patients may present with neurological features [8]. Paraneoplastic syndromes and lytic bony lesions may also be present [6, 9]. As cases are often diagnosed when patients present with advanced disease, prognosis is invariably poor with a median survival of less than a year [10].
The optimal treatment of SCCP has not been clearly established. Oncologists’ experiences suggest SCCP is sensitive to chemotherapy and radiotherapy [4]. In individual cases with a high acinar component, androgen deprivation treatment (ADT) may be added to treat the conventional component, however the SCCP component does not usually benefit from ADT [6, 7]. Novel agents are currently being investigated in patients with neuroendocrine prostate cancer, such as aurora kinase A (AURKA) inhibitors [4, 9].
In conclusion, it is important to recognize SCCP as a rare variant and not mistake it for a high-grade Gleason 5 pattern adenocarcinoma. Differentiating the separate forms of prostate cancer (adenocarcinoma, pure SCC and mixed) is essential to selecting the most effective form of treatment. Immunohistochemistry is useful for confirmation of the diagnosis. Histologically, the percentage and grade of the acinar component in the pathology report may be valuable for individual case management with androgen deprivation.
SCCP is an aggressive form of malignancy. Clinicians should be aware that it metastasizes early and, unlike conventional prostate adenocarcinoma, serum PSA levels do not correlate with disease severity. Further studies on novel targeted therapies may achieve better clinical outcomes for patients with this aggressive type of prostate cancer in the future.
Conflict of interest statement
None declared.
FUNDING
No funding to declare.



