-
PDF
- Split View
-
Views
-
Cite
Cite
Siddharth Pahwa, Susmit Bhattacharya, Siddhartha Mukhopadhyay, Ashok Verma, Aorto-esophageal fistula: successful open surgical management of two cases, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa114, https://doi.org/10.1093/jscr/rjaa114
Close - Share Icon Share
Abstract
An aorto-esophageal fistula (AEF) is a rare yet life-threatening cause of upper gastrointestinal bleeding. We report our experience with open surgical management of two cases of AEF. Both cases presented with almost identical presentations: hematemesis and hemodynamic instability. The aorta in the first patient was normal; the defect was small and was repaired with a Dacron patch. The second patient had an aneurysmal aorta, which was replaced with a Dacron graft. Both cases were performed under partial bypass. The esophageal rent in both patients was debrided, primarily closed and buttressed with a vascularized intercostal pedicle. Nonavailability of endovascular personnel and equipment along with hemodynamic instability of the patient influenced our surgical strategy. Long-term follow-up of these patients is necessary to analyze the outcomes of our surgical repair.
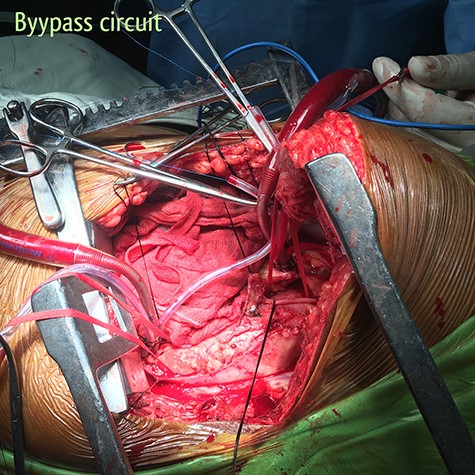
Partial bypass circuit. Outflow cannula is located in the aortic arch between the left common carotid artery and the left subclavian artery; inflow cannula is located in the descending thoracic aorta just above the diaphragm.
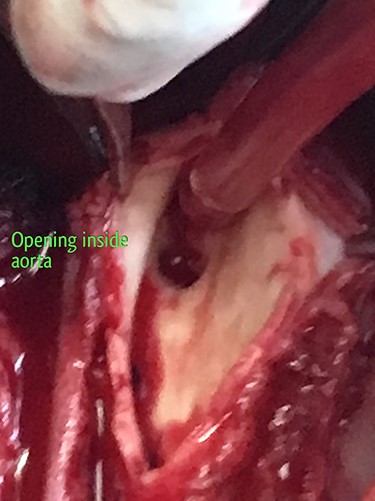
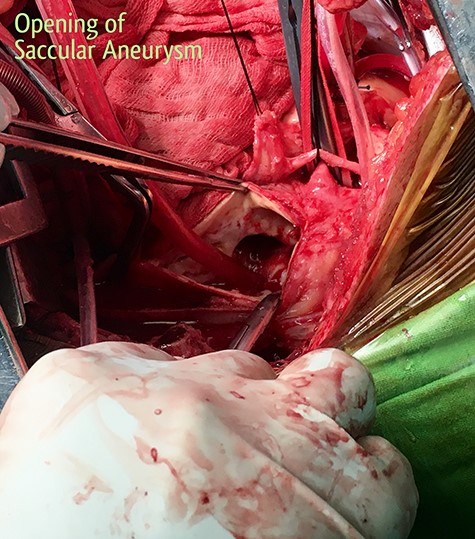
2 × 2 cm opening of the fistula inside the descending thoracic aorta.
INTRODUCTION
An aorto-esophageal fistula (AEF) is a rare yet life-threatening cause of upper gastrointestinal (UGI) bleeding. The disease typically has a high mortality rate, with few reported survivors [1]. The common causes of AEF are thoracic aortic aneurysms, trauma, carcinoma esophagus and radiation [2]. The clinical characteristics of AEF are unique and a presumptive diagnosis maybe made at presentation. It classically presents with the triad of mid-thoracic pain, sentinel arterial hemorrhage and exsanguination after a symptom-free interval (Chiari’s triad of aorto-esophageal syndrome) [3]. The diagnosis of an AEF warrants urgent surgical management. We report our experience with open surgical management of two cases of AEF.
CASE SERIES
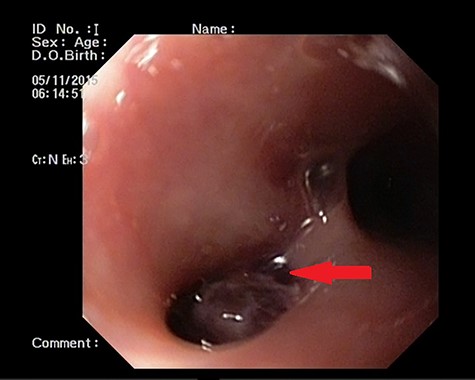
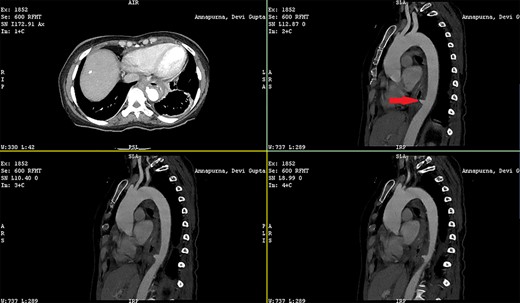
Patient 1: a 52-year-old woman, non-diabetic, non-hypertensive, was referred to the emergency with a history of large-volume, frank hematemesis (four episodes in 3 days). Positive history included recurrent cough for the past 1 year and low-grade fever for 3 months. No history of dysphagia, jaundice, abdominal distension, pain abdomen or altered sensorium. No history of chronic medications (oral anticoagulants or anti-platelets), analgesic intake or any substance abuse. She had received four units of packed red cells at an outside facility prior to admission. She was conscious, oriented, hemodynamically labile (heart rate: 110/min; BP: 86/60 mm Hg) with mild fever (99°F). General survey revealed obvious pallor. All other system examinations were within acceptable limits. Her hemoglobin (Hb) was 7.3 gram%, total leukocyte count (TLC) was 6400 cells/mm3, total bilirubin was 1.31 mg/dl and total protein was 4.75 gram%. An urgent UGI endoscopy revealed an opening in the posterior wall of the lower third of the esophagus, with a diverticulum. Inflammatory changes were noted in the diverticulum (Fig. 1). Contrast-enhanced computed tomogram (CECT) revealed erosion of the pouch into the thoracic aorta (Fig. 2), and a diagnosis of AEF with acute UGI hemorrhage was made.
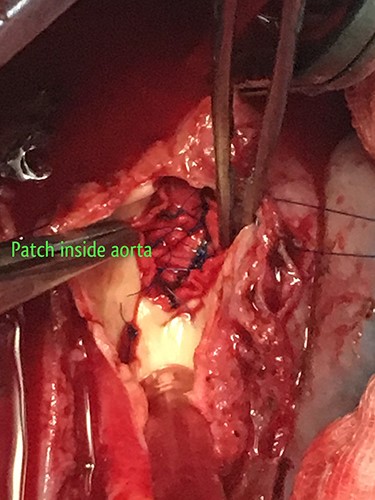
The patient was resuscitated with packed red cells and definitive surgical management was emergently undertaken. A left posterolateral thoracotomy was performed, and pus was found in the pleural cavity and posterior mediastinum. After debridement of the chest cavity, partial bypass was instituted by cannulating the aortic arch in between the left common carotid artery and the left subclavian artery, and connecting this via the cardiopulmonary bypass machine to another cannula in the descending thoracic aorta just above the diaphragm (Fig. 3). The aorta was cross-clamped above and below the suspected fistulous region and opened longitudinally. A 2 × 2 cm opening was seen in the wall of the aorta, adjoining the esophagus (Fig. 4), and this was internally repaired with a Dacron patch (Fig. 5), and the aorta was primarily closed. The esophageal opening was debrided and then repaired with interrupted polypropylene sutures, superimposed with a vascularized intercostal muscle pedicle (Fig. 6). Bypass was successfully discontinued and a gastrostomy and jejunostomy were created. She remained on broad-spectrum antibiotics and the rest of her postoperative course was uneventful.
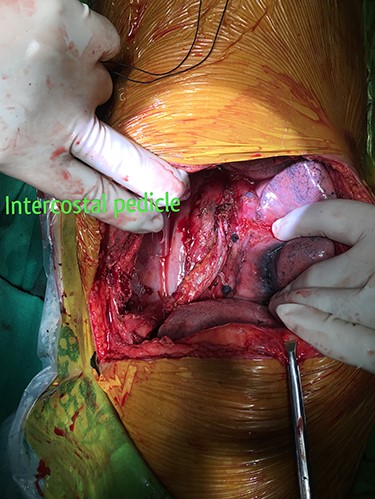
Vascularized intercostal pedicle harvested to re-enforce the primary closure of the esophageal opening of the fistula.
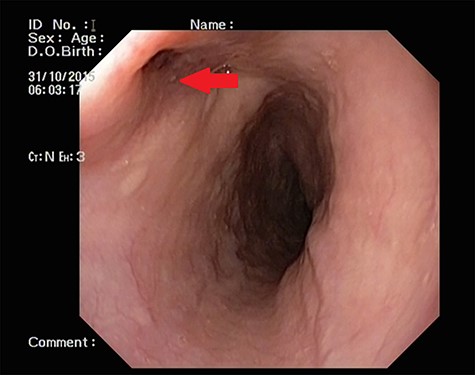
UGI endoscopy showing a large depressed ulcer located in the middle third of the esophagus; a diverticulum was seen in the lower end of the ulcer (red arrow).
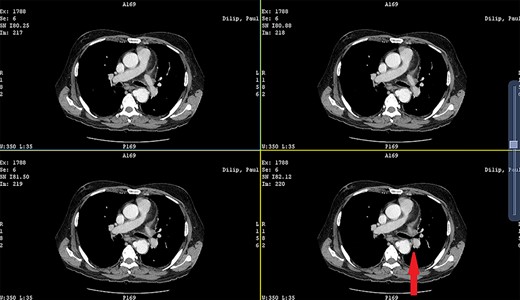
CECT showing the descending thoracic aortic aneurysm; the red arrow shows the communication of the aneurysm with the esophagus.
Patient 2: a 48-year-old hypertensive, non-diabetic gentleman, presented to the emergency with one episode of massive hematemesis. There was no history of dysphagia, jaundice, abdominal distension, pain abdomen or altered sensorium. Past history was significant for percutaneous coronary intervention with two drug-eluting stents performed 3 years ago, for which he was on aspirin. He had normal mentation, his BP was 90/60 mm Hg and his heart rate was 120/min. A general survey revealed pallor and rest of the systemic examination was normal. His Hb was 6.1 gram% and TLC was 6300 cells/mm3. Liver function tests, coagulation parameters, renal function and electrolytes were normal. Chest X-ray was unremarkable. He was initially resuscitated with intravenous fluid, packed red blood cells and a proton-pump inhibitor infusion. An urgent UGI endoscopy revealed a large depressed ulcer with a red spot located in the middle third of the esophagus. A diverticulum was seen in the lower end of the ulcer (Fig. 7). An endoscopic clip was applied to the margin for ease of identification. CECT thorax revealed a 6 cm saccular thoracic aortic aneurysm distal to the left subclavian artery, eroding into the esophagus (Fig. 8).
Surgery was emergently undertaken. A posterolateral thoracotomy was performed, and the aneurysmal segment of the aorta was seen originating 6 cm distal to the left subclavian artery. A partial bypass circuit was created, similar to the one described earlier. After cross-clamping the aorta both proximally and distally, the aneurysm sac was opened (Fig. 9). The aneurysmal segment of the aorta was replaced with a Dacron graft, taking care to preserve as many intercostal arteries as possible, while suture ligating the others (Fig. 10). The esophageal opening of the fistula was debrided and repaired with interrupted polypropylene sutures, superimposed with a vascularized intercostal muscle pedicle. The patient was successfully separated from the partial bypass, and a gastrostomy and jejunostomy were created. He was placed on broad-spectrum antibiotics postoperatively. He recovered well and had an uneventful post-operative course.
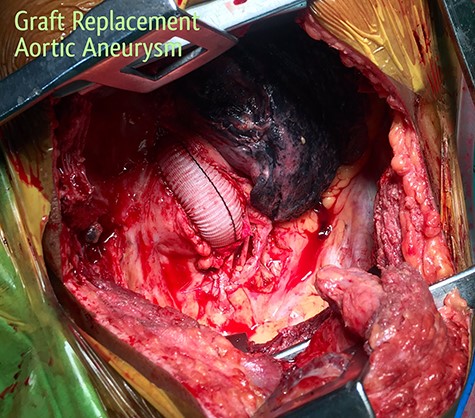
Replacement of the aneurysmal segment of the descending thoracic aorta with a Dacron graft, under partial bypass.
DISCUSSION
The main causes of an AEF are thoracic aortic aneurysms, foreign body ingestion and advanced esophageal malignancy [3]. In addition, some cases of AEF may occur as a complication after surgical prosthetic repair of an aneurysm or thoracic endovascular aneurysm repair (TEVAR) [4]. Many pathophysiologies have been proposed for the exact mechanism of AEF including, esophageal ischemia due to esophageal artery occlusion, mechanical compression by a large aneurysm and secondary erosion, increased pressure in the posterior mediastinum, primary infection or inflammation of the aneurysmal wall and radial force of an endovascular graft against the native aortic wall in cases of TEVAR [5].
The diagnosis of an AEF usually warrants urgent, if not emergent intervention, especially if accompanied by bleeding and hemodynamic instability. The management consists of mandatory control of the bleeding site with definitive aortic treatment, accompanied by methods to stop continuous contamination of the aortic prosthesis through the fistula [6]. Conservative medical treatment results in poor survival [3, 5]. Radical esophagectomy is usually the most effective strategy to control contamination, but primary closure of the perforated esophagus has been tried and resulted in a certain amount of success [6].
In situ reconstruction of the aorta, with either cardiopulmonary bypass or partial bypass has been reported as a suitable strategy to control the bleeding site [7]. Increasing reports have focused on the role of endovascular management of the thoracic aorta in hemodynamically unstable patients in order to stabilize their condition [8, 9]. Endovascular insertion of a stent graft may play a pivotal role in the cessation of hematemesis and in the stabilization of hemodynamic status, and TEVAR followed by early open repair may result in better outcomes [9]. However, in a setting with no endovascular surgeon or stent grafts available, open surgical repair was our only option. In an emergent scenario with a hemodynamically unstable patient, primary repair of the esophageal opening and buttressing it with a vascularized intercostal muscle flap was deemed the most optimal surgical strategy at that time. This technique, though less popular now, has been reported in the past with some success [10].
Both the cases reported here had almost identical presentations. In the first patient, the descending thoracic aorta was normal, and no apparent cause for the AEF was evident. The second patient had an atherosclerotic aortic aneurysm that ruptured in to the esophagus. Long-term follow-up of these patients is necessary to analyze the outcomes of our surgical repair.



