-
PDF
- Split View
-
Views
-
Cite
Cite
Bobak Rasouli, Kristine Pederson, Marshall F Wilkinson, Mohammad Zarrabian, Peripheral leg ischemia detected via intraoperative neurophysiological monitoring during a multilevel complex anterior and posterior operation, Journal of Surgical Case Reports, Volume 2020, Issue 5, May 2020, rjaa049, https://doi.org/10.1093/jscr/rjaa049
Close - Share Icon Share
Abstract
Intraoperative neurophysiologic monitoring is a technique utilized during spinal operations to minimize sensory and motor function morbidity. We herein report a case of a 73-year-old female with renal cell carcinoma and metastatic involvement of the cervical and thoracic spine, who underwent a multilevel complex anterior and posterior operation. Neurophysiological monitoring was able to localize the lower limb ischemia utilizing somatosensory evoked potentials. This prompted intraoperative investigation of the peripheral ischemia, and the patient was found to have an Angio-Seal device embolus in the right popliteal artery that dislodged from the right femoral artery.
INTRODUCTION
The advantages of intraoperative neurophysiologic monitoring (IONM) have made it an increasingly popular monitoring modality to reduce adverse outcomes for many spine surgeries. In particular, complex multistage or multilevel operations with ongoing spinal cord compression or requiring deformity correction frequently utilize the IONM. Such procedures are thought to benefit from IONM surveillance incorporating motor evoked potential and somatosensory evoked potential (SSEP) monitoring. During the operation, alterations in the neurophysiological signals are used to warn the surgeon of conditions with the potential to damage the spinal cord or components of the sensory or motor pathways. In a 2010 survey of 105 Canadian spine surgeons [1], 62.1% of respondents reported using a form of electrophysiologic monitoring during some spinal procedures. Furthermore, most respondents of the survey indicated that neurophysiologic spine monitoring should be used for the reduction of major deformities. We present a unique case where neurophysiological monitoring during a complex spine operation detected limb ischemia rather than changes in spinal cord function.
CASE REPORT
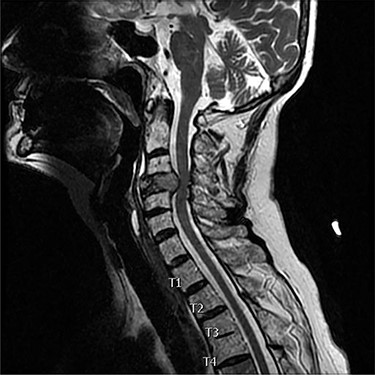
A 73-year-old female presented with neck pain and shooting pain down her left arm with associated paresthesias. Axial and sagittal imaging in the form of magnetic resonance imaging and computed tomography (CT) revealed a pathological fracture of C4, with extraosseous tumor extension from the vertebral body and a left lateral mass causing severe cervical spine stenosis (Figs 1 and 2). Thoracic imaging demonstrated a dorsal expansile osteolytic lesion at T8 with concomitant severe spinal canal stenosis. Following a standard work up of the lesion, it was determined that the patient had a renal cell carcinoma. A CT angiogram showed the cervical lesions received significant blood supply from the left vertebral artery.
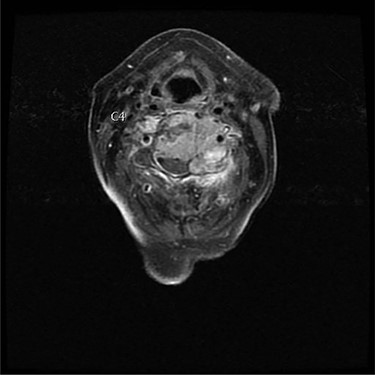
Axial view of C4 tumor showing encasement of left vertebral artery.
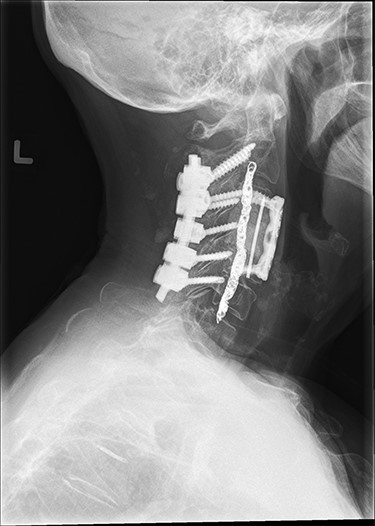
Approximately 24 hours prior to spine surgery, the patient had undergone an interventional radiology procedure to embolize the left vertebral artery. As part of the procedure, an Angio-Seal device was placed in the femoral artery for closure. The next day, stage one of the spine surgery consisted of a posterior C2–C6 (Fig. 3) and a T7–T9 decompression and instrumented fusion while the patient was in the prone position. After the completion of stage one, the patient was moved to a supine position using a Jackson table. During stage two, the patient underwent an anterior C3–C5 (Fig. 3) instrumented fusion with a C4 vertebrectomy.
During the final 10 minutes of stage one closure, the right lower SSEP amplitude was decreased by ~40%, which did not meet our established SSEP warning criteria of ≥50% signal decrement. Neurophysiologic monitoring was then paused to facilitate repositioning of the patient from prone to supine for stage two of the spine surgery. The repositioning period lasted 35 minutes and volatile anesthetic was utilized during this time. Following the repositioning, the neurophysiologic monitoring was resumed and found that there was a complete loss of the right lower SSEPs in all channels. The left lower and bilateral upper SSEPs were unchanged and upon visual inspection the right lower stimulating and recording electrodes were still intact. During the visual inspection, skin discoloration of the right lower limb was also noted. Next, the right lower SSEP stimulating electrode was moved from the ankle to the popliteal fossa, yielding a robust cortical response. This result strongly suggested distal leg arterial ischemia, at which point the on-call vascular surgeon was consulted. A Doppler scan was conducted at the right popliteal fossa, where a pulse was appreciated but was much weaker than the left popliteal pulse. A right femoral embolectomy was performed simultaneously with the anterior cervical decompression and instrumented fusion. The embolus was found to be the anchor of the Angio-Seal device. The vascular surgeon recommended full anticoagulation to prevent reocclusion of the femoral artery; however, due to the risk of compressive hematoma formation in the thoracic and cervical spine this could not be undertaken. Unfortunately, reocclusion of the vessel resulted in an above knee amputation due to ischemia. Two years postoperatively, the patient has been able to ambulate with no local reoccurrence of the metastases.
DISCUSSION
When discussing the use of IONM in spine operations, it is typically considered a technique employed for the assessment of spinal cord and nerve root patency. Rizzo et al. [2] concluding that IONM is highly sensitive and specific for detecting and preserving spinal cord and nerve root function during spine surgery. To the best of our knowledge, it has yet to be documented where the use of IONM has successfully located and diagnosed peripheral limb ischemia during spine surgery.
While the detection of peripheral neuropathy during spinal procedures is not considered the primary purpose for IONM, these techniques have been utilized for this purpose in other surgical procedures. One such procedure was an aortic aneurysm operation by Arikan et al [3]. They reported that intraoperative PtiO2 monitoring combined with IONM offered early and reliable detection of ischemia. Herrera-Perez et al. [4] utilized IONM in peripheral nerve procedures. They reported all 11 peripheral nerve schwannomas were successfully resected from the main trunk of the nerve with no incidents. Similarly, Kwok et al. [5] utilized intraoperative neuromonitoring while removing brachial plexus tumors, concluding its use minimized risks and also increased the likelihood of achieving a good clinical outcome.
This case has challenged the conventional use of IONM during spinal operations. Its use in preventing spinal cord and nerve root damage intraoperatively is well documented; however, we show further utility for IONM use during spinal operations. This is especially valid during complex cases where patient positioning, bleeding and vascular integrity are also of concern.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- ischemia
- limb ischemia
- renal cell carcinoma
- femoral artery
- intraoperative care
- intraoperative complications
- popliteal artery
- leg
- morbidity
- neurophysiology-biologic function
- embolism
- motor function
- intraoperative neurophysiological monitoring
- somatosensory evoked potentials
- thoracic spine
- lower limb ischemia
- ischemia, peripheral
- medical devices
- spinal procedure



