-
PDF
- Split View
-
Views
-
Cite
Cite
Sidney Parker, Sarah Bouayyad, Jonathan Tam, Nisheeth Kansal, Vish Bhattacharya, Emphysematous cystitis: a cause of hematuria post angioplasty, Journal of Surgical Case Reports, Volume 2020, Issue 2, February 2020, rjz401, https://doi.org/10.1093/jscr/rjz401
Close - Share Icon Share
Abstract
Emphysematous cystitis (EC) is a rare, severe, urinary tract infection caused by gas-producing bacteria. It is characterized by the accumulation of air inside the bladder wall and/or lumen and is common among elderly diabetic females. This case of a 90-year-old female, who underwent bilateral iliac angioplasty and stenting, is the first published correlation between iliac angioplasty and the development of EC. The endovascular procedure performed was a success, but later on, she was confused and developed frank haematuria with a fall in haemoglobin levels. CT revealed severe EC with abundant gas tracking outside of urinary bladder into the extraperitoneal space. She was catheterized and empirical antibiotics were started and converted based on the culture sensitivities. Frequent follow-up ensured the resolution of a conservatively managed case. It is essential to increase the awareness of EC associated with angioplasties among the healthcare staff, particularly those undertaking vascular procedures.
INTRODUCTION
Emphysematous cystitis (EC) is described by the accumulation of air inside the bladder wall and/or lumen. It is one of the infrequent variety of a urinary tract infection (UTI) caused by gas-producing bacteria. EC is a rare but probably critical condition with a mortality rate of 7.4% [1]. It is more frequently seen in elderly, diabetic females with an infective organism [1–4] and immunocompromised people [5, 6, 7]. Apart from its association with diabetes mellitus (DM) [1–4, 7, 8], it has evidence of being associated with other medical conditions such as liver diseases [1, 5], cardiac diseases [3, 6, 9], carcinomas [6] and prostatic conditions [1, 7, 8], which may or may not have undergone any surgical procedures.
This case is the first published correlation between iliac angioplasty and the development of EC.
CASE REPORT
A 90-year-old female with bilateral leg ulcers and rest pain had presented at the department for bilateral iliac angioplasty and stenting. The multidisciplinary team deemed her as not fit for an open procedure due to multiple comorbidities including, type 2 DM, chronic kidney disease, hypertension, hypothyroidism and glaucoma. The lesion was also a TransAtlantic Inter-Society Consensus (TASC) Type B lesion, which would be more suitable for an endovascular procedure. Preoperatively the patient had an arterial duplex of the aortoiliac segment and a magnetic resonance angiogram (MRA) demonstrating 70% stenosis of the offending vessel (Fig. 1).
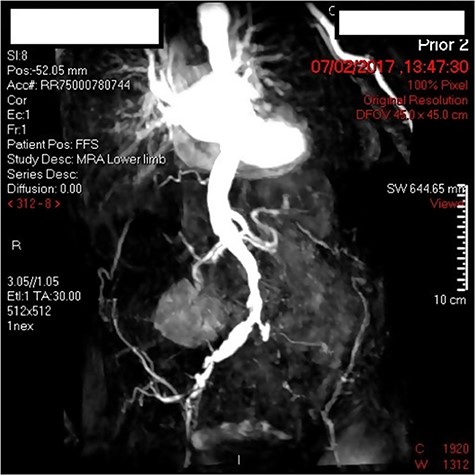
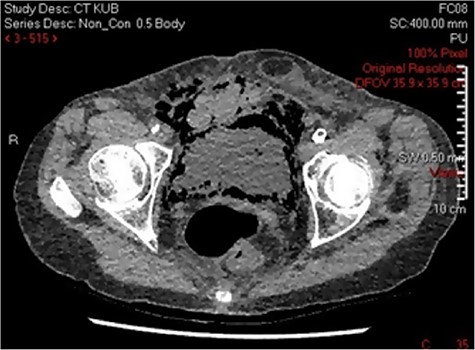
Three days post-procedure, the patient was confused and developed frank haematuria with a fall in haemoglobin from 124 to 107 g/L. A computed tomography (CT) scan of abdomen and pelvis demonstrated severe EC with abundant gas tracking outside of urinary bladder into the extraperitoneal space, extending to the anterior abdominal wall and inguinal orifice on the right, and obturator foramen on the left (Fig. 2). The urology on-call team recommended catheterization and treatment with empirical intravenous Piperacillin with Tazobactam for 5 days. A septic screen was undertaken including, blood tests and urine, wound, sputum and blood cultures. Klebsiella pneumoniae has cultivated in the urine culture, and subsequently, the microbiologist advised on converting drug regime to oral Trimethoprim based on culture sensitivities. The patient was discharged on the completion of the 14-day course of antibiotics.
During the follow-up phase, an outpatient flexible cystoscopy was undertaken a month following the EC. It demonstrated mild diffuse submucosal haemorrhagic areas of the bladder with catheter-associated cystitis. The patient’s catheter was removed and she made a complete urological recovery.
The patient was seen in the vascular clinic six weeks following the post-operative episode of EC, with non-healing right leg ulcers. Two weeks later, she subsequently had the right common femoral artery and superficial femoral artery angioplastied to 5 mm with good results. The left superficial femoral artery was dilated to 5 mm with a further angioplasty 3 weeks later. Subsequent clinical follow-up demonstrated a good response to treatment with improvement in her ulcers and symptoms.
DISCUSSION
Three types of UTIs contribute to the formation of gas within the urinary system; naming EC, emphysematous pyelitis and emphysematous pyelonephritis. The prevalence of EC among females and males is nearly 2:1 [1, 10]. EC is often asymptomatic [1, 3], yet clinical features such as abdominal pain [2, 5, 7–9], fever [2, 5, 7], haematuria [4, 7–9], painful/burning micturition [2, 4] and voiding dysfunctions [7–9] may occur defining the severity of the condition. The most frequent organisms that cause EC are Escherichia coli [1–4, 6–8, 10] and K. pneumoniae [1, 5, 6, 10]. In the case we present, K. pneumoniae (the second most common causative organism) was isolated in the patient’s urine cultures.
We have presented an unusual case of a lady developing EC following common iliac angioplasty. Concerning our case, diabetic women are at high demographic risk for developing EC. The exact pathophysiology driving EC is not yet fully understood. However, it is known that DM favours bacterial colonization of the urinary tract by bacteria due to impaired leukocyte function. The high concentrations of glucose in the urinary bladder increase the likelihood of bacterial fermentation, which is required for emphysematous UTI to occur [6]. The existing literature reveals the association of comorbidities, specific virulence factors in pathogens and impaired gas transfer (due to inflammation or obstructive procedures), with EC [10].
Differentiation of EC from other conditions is mainly via X-rays, ultrasonography, and CT scan. Radiographic findings of EC include speckled areas of high radiolucency in the zone of the urinary bladder, which changes with the patient’s position. The air in the bladder wall may visualize as a “beaded necklace” appearance denoting irregular thickening of the non-dependent mucosal surface [10]. Ultrasonography can exhibit echogenic gas within the bladder wall with a dark shadowing artefact [10]. CT scans permit early detection of intraluminal/intramural gas. It assists in detecting the severity and extent of the condition in addition to evaluating other causes of intraluminal gas [2–4, 8–10]. CT scanning is the most preferred and accurate diagnostic imaging technique for this condition.
The occurrence of EC as a post-operative complication is rare. But several incidences have reported resulting in EC following implantation of left ventricular assist device [6] and coronary angiogram [9]. Our case is the first reported case to acquire EC following iliac angioplasty and stenting. We posit that bladder wall pneumatosis could be more likely in those with relative ischaemia of the bladder (in this case, an arteriopath post iliac angioplasty) via an analogue proposed similarly to the pathogenesis of pneumatosis intestinalis. Gas produced by bacterial fermentation could infiltrate the cystic wall through breaks in the mucosal surface. Indeed a recent case presented saw ileal and bladder wall pneumatosis on CT following a coronary angiogram and a catastrophic vascular event in a non-diabetic patient. They proposed a similar mechanism of action [9].
This case of developing EC following common iliac angioplasty contributes further to literature and proposes that microscopic bladder infarction from a vascular procedure may exacerbate pre-existing infections in at-risk patients and precipitate cases of EC. Although the incidence of EC following endovascular procedures is rare, its awareness would reduce the postoperative mortality rate through early detection and treatment. This case also highlights a rarer differential diagnosis to post angioplasty haematuria. Centers that have high volumes of angioplasty should be aware of this potential cause of frank haematuria and the effective method of conservative management, which we applied.
References
- stents
- diabetes mellitus
- urinary tract infections
- hematuria
- angioplasty
- follow-up
- vascular surgical procedures
- bacteria
- urinary bladder
- empirical antibiotic therapy
- hemoglobin measurement
- emphysematous cystitis
- older adult
- iliac artery angioplasty
- bladder wall
- hematuria, gross
- extraperitoneal space
- endovascular procedures



