-
PDF
- Split View
-
Views
-
Cite
Cite
Emily J Burns, Peter M Smith, Matthew Liew, Proximal migration of a ‘double J’ ureteric stent in a patient with a staghorn calculus, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa527, https://doi.org/10.1093/jscr/rjaa527
Close - Share Icon Share
Abstract
Ureteric stents are widely used for the management of obstructive uropathy and intraoperative identification of the ureters. Despite undergoing numerous modifications since their introduction in 1967, they are frequently associated with complications ranging from irritative symptoms to migration of the stent. Proximal migration of ureteric stents is a relatively uncommon occurrence, with a reported incidence of 1–4.2%; it is usually associated with inappropriate stent length, poor positioning or incorrect deployment of the stent. Here we discuss an interesting case of a patient who unfortunately suffered proximal ureteric stent migration associated with pelvico-ureteric junction obstruction, despite appropriate stent choice, adequate deployment and confirmation of correct positioning. This complication likely occurred secondary to mechanical disruption of the stent caused by the presence of a large staghorn calculus within the renal pelvis.
INTRODUCTION
Ureteric stenting is one of the most commonly applied urological techniques, particularly since the introduction of the ‘double J’ stent by Finney in 1972 [1]. Despite their versatility in the management of obstructive uropathy and intraoperative identification of the ureters, they are not without complications. Patient morbidity following stent insertion is high; a study by Joshi et al. [2] found that many patients experience irritative symptoms, such as increased urinary frequency, and discomfort [3]. Less commonly, migration of ureteric stents may occur; this is either distal or proximal, the latter being less common with a reported incidence of 1–4.2% [4].
There are many factors influencing stent migration including the shape of the stent; in 1972, barbs were added to the original stent to prevent distal migration [5, 6, 7]. These styles were superseded by pigtail designs, initially applied to the distal end only, through to the ‘double J’ stent used today [6]. Another factor affecting migration is the length of the stent; required stent length may be predicted using the patient’s height, through computed tomography (CT) measurements or using guidewire techniques [7]. Directly measuring the ureter using a ureteric catheter is not recommended as it is associated with increased radiation exposure and the cost implications of an additional procedure [8]. Whilst stents that are too short may migrate, stents that are too long may cause mechanical irritation to the bladder and therefore have worse quality of life outcomes [8]. Stent material also has an impact; stiffer materials with greater memory such as polyurethane are less prone to migration than softer materials, such as silicone [9]. Patient factors that could affect stent positioning including the length of time a stent is indwelling (usually 6 months depending on manufacturer recommendations) and renal movement during respiration [4, 7]. Finally, surgeon factors, including poor positioning and incorrect deployment, may lead to stent migration. The use of fluoroscopy at the time of insertion aids identification of malposition and subsequent correction [7].
CASE REPORT
A 72-year-old male with a history of bleeding per rectum underwent an outpatient colonoscopy, which demonstrated a large villous tumour occupying the caecal pole. A staging CT thorax, abdomen and pelvis was subsequently performed confirming the presence of tumour with liver metastases and an incidental 33 × 21 mm calculus in the right renal pelvis with associated hydronephrosis (Fig. 1).
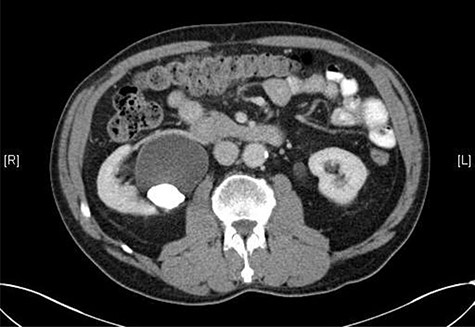
CT scan showing a staghorn calculus in the right renal pelvis with associated hydronephrosis.
The case was discussed in the colorectal multidisciplinary team (MDT) meeting and the consensus for management of the caecal tumour was a laparoscopic right hemicolectomy and for uro-radiological review of the imaging to discuss the management of the staghorn calculus.
Following review by the uro-radiology team, a mercaptoacetyltriglycine (MAG3) renogram was requested to assess the split function and drainage of the kidneys—found to be 54% (right kidney) and 46% (left kidney). The right kidney showed significant isotope remaining in the renal pelvis at 30 min suggesting pelvico-ureteric junction obstruction (PUJO). The patient’s renal function was stable with an estimated glomerular filtration rate range of 80–89, and urea and creatinine within normal limits. It was suggested that a ureteric stent was inserted during the laparoscopic right hemicolectomy to decompress the right kidney and optimize renal function prior to adjuvant chemotherapy.
Seven months after the patient’s initial presentation, they underwent a laparoscopic right hemicolectomy; intra-operatively, a 6Ch/24 cm stent was inserted into the right ureter by the colorectal surgeon who had good experience of ureteric stent placement. The position of the stent was confirmed with cystoscopy and retrograde pyeloureterogram (Fig. 2).
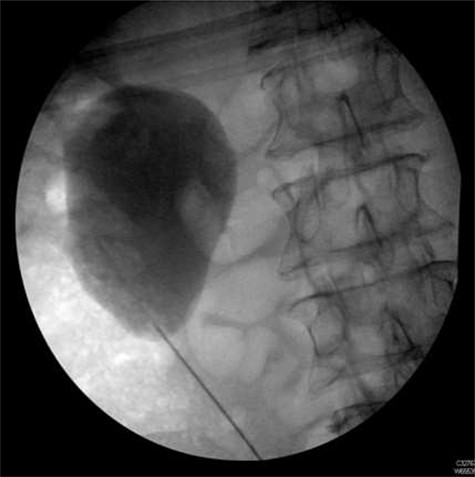
Retrograde pyeloureterogram confirming position of right ureteric stent.
On day three post-procedure, the patient had an abdominal X-ray, which demonstrated correct positioning of the stent (Fig. 3). They were discharged from hospital on day five with planned follow-up for cystoscopic stent removal under local anaesthetic in 6 weeks.
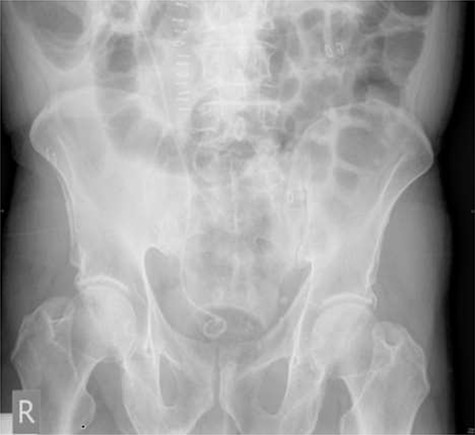
Post-operative plain abdominal X-ray demonstrating acceptable right ureteric stent position.
Removal of the ureteric stent was delayed as the patient was undergoing chemotherapy and it was felt unnecessary instrumentation of the urinary tract when immunocompromised may lead to complications.
Following completion of chemotherapy, 12 weeks after stent insertion, the patient attended clinic for stent removal however, the stent could not be visualized on flexible cystoscopy. An abdominal X-ray showed proximal migration of the stent with the proximal end of the stent appearing to wrap around the aforementioned staghorn calculus (Fig. 4).
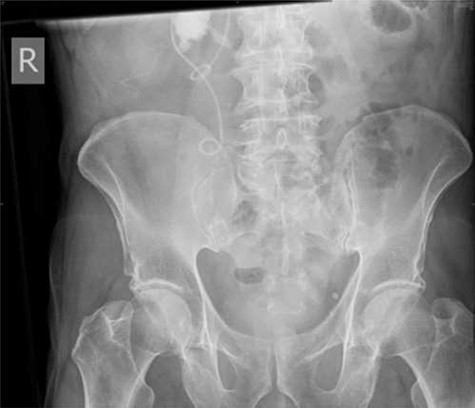
Plain abdominal X-ray performed at follow-up demonstrating proximal ureteric stent migration.
The stent was left in situ and the case was discussed in the urology MDT meeting. The consensus was that percutaneous nephrolithotomy (PCNL) and stent removal should be performed.
The patient underwent PCNL, ureteric stent removal and insertion of nephrostomy 6 months after its initial insertion. There were no intraoperative complications, however a post-operative nephrostogram demonstrated ongoing PUJO, likely secondary to the prior calculus. The nephrostomy was changed and further urology follow up arranged.
DISCUSSION
Whilst there are many factors that have been identified that can lead to ureteric stent migration, these do not apply in this case. The stent was of appropriate length, correctly deployed and satisfactory positioning was confirmed. Additionally, the degree of migration was vast, making removal of the stent difficult without performing PCNL.
We hypothesize that the presence of the large staghorn calculus led to mechanical disruption of the stent. It is possible that ventilatory and general movements resulted in rotation of the calculus; thus causing a cog-wheel effect responsible for the subsequent migration of the stent.
A similar phenomenon and mechanism of migration is seen in patients with a pacemaker—Twiddler’s syndrome. This is a rare complication arising from mechanical manipulation of the pacemaker device by the patient, leading to displacement of the pacing leads from the ventricles [10].
This theory would explain why the stent migrated, despite being correctly placed and its position confirmed with intra-operative and post-operative imaging.
CONCLUSION
Ureteric stent migration is a well-known but relatively rare complication of ureteric stent placement and there are several proposed theories behind this occurrence. However, we report a case whereby mechanical manipulation of the stent, through the cogwheel effect of a staghorn calculus, has resulted in proximal ureteric stent migration; a phenomena comparable to the migration of pacemaker leads in Twiddler’s syndrome.



