-
PDF
- Split View
-
Views
-
Cite
Cite
A Kriger, S Berelavichus, D Gorin, G Galkin, M Yukina, I Ilovayskaya, V Zektser, N Nuralieva, A R Kaldarov, Successful surgical treatment of a patient with primary insulinoma of the liver, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa519, https://doi.org/10.1093/jscr/rjaa519
Close - Share Icon Share
Abstract
Insulinoma is the most frequent functional neuroendocrine tumor of the pancreas, which causes organic hyperinsulinism with severe hypoglycemia. There are no cases of primary insulinoma of the liver described. A 63-year-old female was admitted to our center presenting symptoms of severe hypoglycemia due to confirmed endogenic hyperinsulinism. None of the performed diagnostic procedures could reveal a pancreatic tumor. However, a pathologic mass in the sixth segment of the liver was detected. We performed arterial calcium stimulation that showed increased levels of insulin and c-peptide in almost all stimulated arteries. The highest and most prolonged peaks were detected at the points of the common hepatic artery and the right hepatic artery. After the surgical removal of the liver tumor, the blood glucose level was stabilized within the normal range. Post-operative pathomorphological investigation confirmed the diagnosis of a neuroendocrine tumor. The long-term survival results show correct treatment tactics without any signs of disease recurrence.
INTRODUCTION
Insulinoma is the most frequently occurring hormone-producing tumor of the pancreas, with malignancy signs in only 10–15% of cases. There are extremely rare cases of metastatic insulinoma described in the world literature. In about 1% of cases, insulinoma has an extrapancreatic origin and can be located in the splenic hilum, kidney or duodenum [1–3]. Here, we describe a case of successful surgical treatment of a patient with primary insulinoma of the liver.
CASE PRESENTATION
Female, 63 years old, was admitted to our hospital suffering from weakness, fainting and dizziness at a time of hypoglycemia, memory decline and decreased attention. She had experienced episodes of such symptoms during the previous 2 years. Hypoglycemia with a glucose level of 2.0 mmol/l had been detected during one of the episodes. For the next several months, the patient did not take any diagnostic tests. In 2017, the frequency of the hypoglycemic episodes increased. A fasting test was performed 2 years after the first episode in June 2018. The results showed hypoglycemia 0.91 mmol/l (normal range 3.0–5.5 mmol/l), insulin 54.4 mkE/ml (normal range 3–26 mkE/ml) and c-peptide 5,51 ng/ml (normal range 0.79–4,19 ng/ml). The patient was admitted to our hospital in normal condition. Her BMI was 39 kg/m2; total weight gain was 20 kg since the disease onset.
A transabdominal and endoscopic ultrasound also did not reveal any pathological findings in the pancreas. Contrast-enhanced CT and MRI scans of the abdominal cavity were then performed. A tumor of the pancreas was not revealed, although a pathologic mass in the sixth segment of the liver was detected (Fig. 1).
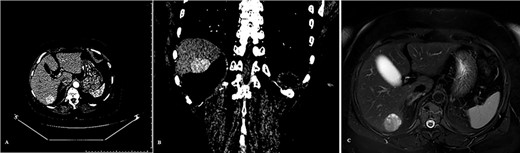
Tumor of the liver detected on the pre-operative diagnostics: (A) CT scan arterial phase frontal projection, (B) CT scan arterial phase frontal projection and (C) MRI scan, axial projection.
Due to being unable to identify a tumor of the pancreas and the pathological finding in the liver, we made a decision to perform arterial calcium stimulation with hepatic venous sampling (ASVS). The analysis showed increased insulin and c-peptide levels in almost all stimulated arteries. Moreover, the highest and most prolonged peaks of insulin and c-peptide levels were detected at the points of the common hepatic artery and the right hepatic artery (Fig. 2). This became the most important finding for further treatment.
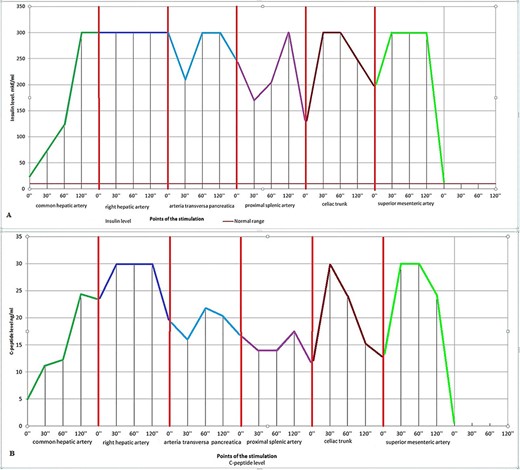
Diagrams of the ASVS. (A) Insulin levels and (B) c-peptide levels.
Therefore, all performed diagnostic procedures (transabdominal US, endoscopic US, CT, MRI and ASVS) could not reveal a pancreatic tumor. However, the lesion of the liver looked like a neuroendocrine neoplasm.
Explorative laparotomy was then performed. Intraoperatively, the pancreatic texture was soft and surrounded by peripancreatic fat tissue without any signs of atrophy or sclerosis. Visualization and palpation of the pancreas did not reveal any tumor. But a soft encapsulated tumor 4 cm in size was found in the sixth segment of the liver. An intra-operative ultrasound was performed. There were no tumors either of the pancreas or of the liver other than the neoplasm described above. Hence, a decision to remove the tumor of the liver was made. This was performed using a bipolar and harmonic ace with minimal organ trauma. The frozen section confirmed a neuroendocrine tumor. The glucose level became 9–10 mmol/l right after the procedure, and no glucose infusions were required.
Histological and immunohystochemical analysis showed a well-differentiated insulin-producing neuroendocrine tumor of grade 2. Ki67 was 4% with positive reaction with Synaptophysin (MRQ-40, Cell Marque): ++; Chromogranin A (DAK-A3, DAKO): ++; CD56 (123C3.D5, Cell Marque): ++ and Insulin (polyclonal, DAKO): +++ (Fig. 3).
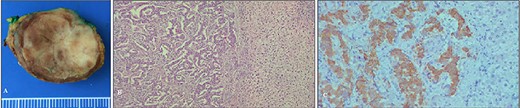
The specimen after surgery: (A) the view of the tumor and (B, C) microphotograph images of the tumor.
Post-operative recovery was uneventful. There were no episodes of hypoglycemia. The range of glucose blood levels was 6–8 mmol/l. The patient was discharged for outpatient checkup on the 14th day after the surgery. During the 2 weeks after the operation, the body weight decreased by 8.5 kg (8.7% of body mass).
Long-term results were assessed on the 6, 9, 12 and 18 months after the surgery. Every 3 months, contrast-enhanced CT and MRI scans were performed. A year after the surgery, a PET/CT scan was also performed. None of the tests revealed any pathological findings or signs of neuroendocrine tumor. At the time of writing, the follow-up period is 20 months after surgical treatment and no episodes of hypoglycemia have been detected.
DISCUSSION
In the described case, we expected to detect an insulin-producing pancreatic tumor which would have been a typical localization of insulinoma. Instead, we found a neoplasm in the liver parenchyma by instrumental diagnostics (CT, MRI, transabdominal and endoscopic ultrasound). We tried to differentiate this lesion either as a benign liver neoplasm, which was not associated with clinical symptoms, or as a metastasis of pancreatic insulinoma, which occurs extremely rarely [4–7].
There have been 92 patients operated with functional pancreatic neuroendocrine tumors in our hospital over the last 11 years, with no cases of metastatic insulinoma. In this case, the neoplasm in a pancreatic parenchyma was not revealed; therefore, we decided to perform arterial calcium stimulation with hepatic venous sampling to confirm the pancreatogenic hyperinsulinism and possibly clarify the localization of the tumor. However, the data received after this analysis surprised us. Pathological insulin and c-peptide levels were observed at all points of stimulation excluding the artery pancreatica dorsalis and the proximal part of the splenic artery. The most unexpected fact was that pathological hormone levels were detected at points of stimulation of the common hepatic artery and, more importantly, the right hepatic artery.
Thus, we proved an organic hyperinsulinism. At the same time, we could not definitely state that it had pancreatogenic origins. Moreover, we hypothesized that a liver neoplasm could produce insulin. Therefore, we decided that the lesion of the sixth segment of the liver was a hormone-producing metastasis of insulinoma without a detected primary tumor. We decided to perform an explorative laparotomy with intra-operative ultrasound. Intraoperatively, no pancreatic tumor was revealed. In this situation, considering the ASVS data with selective pathological levels of insulin and c-peptide from the right hepatic artery, we performed a liver resection. As the follow-up during a year and a half subsequent to surgery shows, the livers tumor was the primary neuroendocrine neoplasm. The chosen tactics of treatment were correct. This was confirmed by control tests (CT, MRI and PET-CT) and the absence of hypoglycemia episodes.
There were an estimated 1067 cases of insulinoma in the study of Stefanini et al. Ectopic insulinomas were revealed in 1% of cases. The lesions were localized in the duodenum, the traits ligament, spleen or perisplenic space. However, the study results might be questionable due to the lack of diagnostics for the year of publication [2, 3].
However, there are sporadic cases of insulin-producing tumors which localized in the kidney, hepatoduodenal ligament, ovary and small pelvis [8–10]. We analyzed the world data (PubMed, MedScape and Cochrane) and could not find any cases of primary insulinomas of liver. There are some published cases about the ectopic pancreatic tissue in the liver. Therefore, we describe the first case of primary insulinoma of the liver. At this moment, follow-up control of the patient’s condition is continued.
CONCLUSION
We present a case of a primary insulin-producing neuroendocrine tumor of the liver without any lesion elsewhere. It was confirmed by radiological and biochemical data and was successfully treated surgically. The short- and long-term survivals confirm that the symptoms were caused by the liver neoplasm.
PATIENT CONSENT
A written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ACKNOWLEDGEMENTS
Marina Chuiko, Elena Dzeranova.
CONFLICT OF INTEREST STATEMENT
The authors declare of no conflicts of interests. The authors alone are responsible for the content and writing of the paper.
Research involving human participants and/or animals: this is a case presentation and no accessory research data is necessary.
FUNDING
The authors has not receive any grants or another funding resource for this paper.



