-
PDF
- Split View
-
Views
-
Cite
Cite
Rahoui Moez, Mrad Daly Khaireddine, Wajih Sahnoun, Alia Zehani, Mokhtar Bibi, Yassine Ouannes, Ahmed Sellami, Sami Ben Rhouma, Yassine Nouira, Symptomatic bladder metastasis of malignant melanoma: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa509, https://doi.org/10.1093/jscr/rjaa509
Close - Share Icon Share
Abstract
Bladder metastasis of cutaneous malignant melanoma is an extremely rare condition, with less than 10 cases reported in the last 30 years in the English literature. Bladder localization is most often asymptomatic, explaining the frequency of cases discovered during autopsy in multi-metastatic patients. We report a case of symptomatic malignant melanoma metastasis to the bladder in a 31-year-old patient.
INTRODUCTION
Bladder metastasis of malignant melanoma is a rare clinical entity, with less than 10 cases reported in the last 30 years in the English literature. Bladder localization is most often asymptomatic explaining the frequency of cases discovered during autopsy. We report a case of symptomatic malignant melanoma metastasis to the bladder in a 31-year-old patient.
CASE REPORT
A 31-year-old man presented with asthenia, intermittent total hematuria and low urinary tract signs. This patient had been diagnosed 3 years ago with malignant melanoma in the right inguinal area and had undergone wide local excision and right inguinal node dissection. He had been treated 6 months ago with Dacarbazine-based chemotherapy for a lymph node recurrence.
His Hematuria was first explored by abdominal ultrasound, showing an irregular wall thickening in the right lateral face and the floor of the bladder measuring 5 × 3 cm (Fig. 1) with a vascularized appearance (Color Dop and a right hydronephrosis. Computed tomography (CT) scan confirmed ultrasound findings and showed metastatic lymph nodes in both inguinal areas and in internal iliac and latero-aortic areas measuring up to 3 cm and a 4 cm mass in the left adrenal gland (Fig. 2). Digital rectal examination finds a fixed bladder floor. He underwent cystoscopy, which revealed a huge solid non-papillary black pigmented lesion located in the floor and in the right lateral face of the bladder hiding the right ureteral meatus (Fig. 3). Monopolar loop electrocautery was used to excise the tumor as a palliative treatment to stop hematuria. Tumor shavings were particularly blackish (Fig. 4). Pathological examination confirmed the diagnosis of malignant melanoma (Figs 5 and 6). The patient was actually treated with chemotherapy for a metastatic malignant melanoma. Although an evaluation CT scan showed significant progression under chemotherapy of the majority of lymph nodes, our patient is no longer complaining of hematuria or low urinary tract signs.
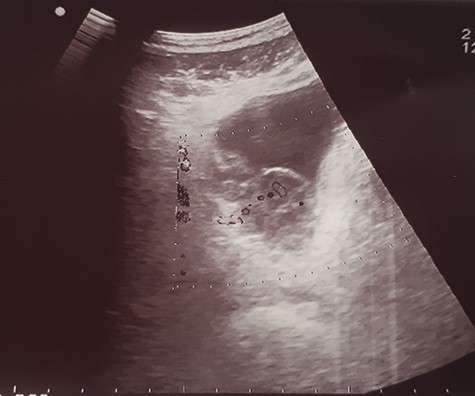
Ultrasound image showing an irregular wall thickening in the right lateral face and the floor of the bladder.
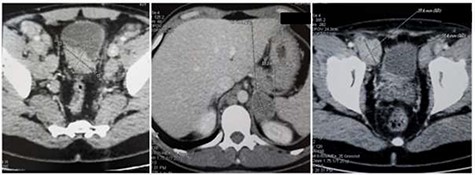
Abdominal CT scan: (a) wall thickening of the bladder, (b) 4 cm mass in the left adrenal gland and (c) 3 cm metastatic inguinal lymph node.
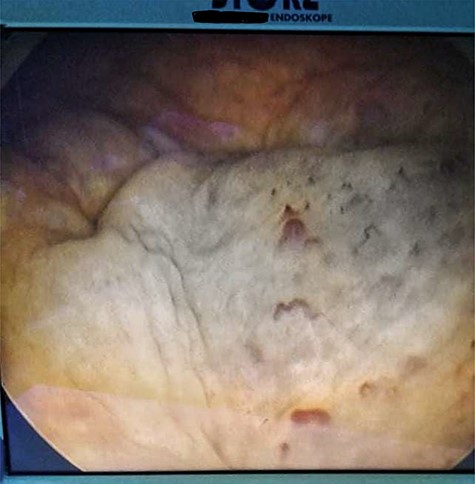
Cystoscopy showing a solid non-papillary black pigmented lesion of the bladder.

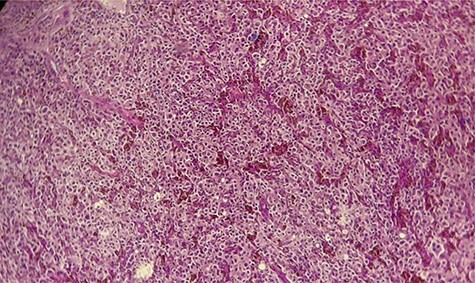
Hematoxylin and eosin staining × 10: diffuse tumor proliferation.
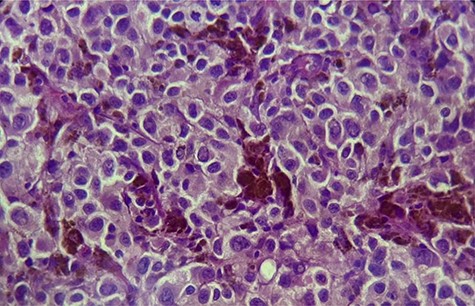
×40: large polyhedral tumor cells, with melanic pigment and enlarged nuclei.
DISCUSSION
Malignant melanoma is a common cancer and extremely aggressive. The genitourinary tract and adrenal gland is a common site of metastasis from malignant melanoma. Most of the metastasis from malignant melanoma is asymptomatic [1]. Bladder metastasis is rarely symptomatic, although approximately 15% of the cases are symptomatic, with hematuria being the most common presentation [2]. The definitive modality for diagnosis was cystoscopy, which demonstrated frequently pigmented and nodular or vegetating lesions in the bladder wall, which was confirmed on biopsy and careful histopathologic evaluation. Adrenal metastases are most often clinically and biochemically silent. CT represents the reference method for diagnosis of adrenal melanoma metastasis [3]. Metastatic malignant melanoma cells usually exhibit variable expression of the common melanoma-associated antigen. In cases where the clinical history of primary melanoma is noted, the diagnosis of bladder metastases is often straightforward and can be confirmed by immunohistochemically stains. In some instances, this variability can create a diagnostic dilemma in which case-special immunostaining is useful. These include antibodies to the gp100, Melan-A/MART-1 or other melanoma antigens [1, 4]. Treatment of patients with systemic melanoma should include careful evaluation for the potential role of different treatments like surgery, radiotherapy and chemotherapy [5]. Transurethral resection of the bladder tumor used for diagnostic purposes could improve the urinary system symptoms. Surgery can be offered to patients with a single metastasis. The use of chemotherapy in the treatment of metastatic melanomas is for palliative purposes. It has not shown efficacy in improving survival [6]. Immunotherapy can be effective in the treatment of metastatic melanoma. Numerous immunotherapy strategies including melanoma vaccines, interferon-alpha and interleukin-2 [IL-2] have been used in the treatment [7]. The prognosis for metastatic malignant melanomas is very poor. Despite the use of new therapeutic modalities, the survival rate of metastatic malignant melanoma patients has not changed considerably over the past three decades. The overall median survival of patients with systemic metastasis from malignant melanoma is about 6–7.5 months, with an estimated 5-year survival rate of 6% [8].
CONCLUSION
Melanoma of the bladder has been very rarely reported. It is typically a secondary recurrence in patients with widespread metastatic melanoma originating from the skin. A detailed patient history and careful examination of the skin are necessary to determine the primary or metastatic nature of the tumor.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONSENT
The authors declare that they have received written consent from the patient to publish this case report.



