-
PDF
- Split View
-
Views
-
Cite
Cite
Obed Rockson, Christine Kora, Abdelbassir Ramdani, Aabdi Basma, Tariq Bouhout, Badr Serji, Tijani El Harroudi, Struma ovarii: two case reports of a rare teratoma of the ovary, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa493, https://doi.org/10.1093/jscr/rjaa493
Close - Share Icon Share
Abstract
Struma ovarii is an extremely rare type of ovarian teratoma distinguished by the unusual presence of thyroid tissue. It is usually a benign condition; however, malignant transformation is sometimes detected. The diagnosis relies on histopathological examination and is infrequently made on routine investigations. We report two cases of struma ovarii: one benign and the other malignant. The first case involved a 27-year-old woman who underwent a right ovarian cystectomy for an incidental ultrasonographic finding of a solid right adnexal mass. The diagnosis of a benign struma was made after histopathological study. The second case involved a 68-year-old woman who underwent a right salpingo-oophorectomy for a right ovarian bulky mass and the histopathological diagnosis was consistent with that of a malignant struma. We examine the challenges involved in the diagnosis and management of this rare entity.
INTRODUCTION
Struma ovarii (SO) is a rare ovarian teratoma composed entirely or predominantly of thyroid tissue. The immense majority are benign, and ~5% are malignant [1]. It often presents with nonspecific symptoms and can mimic ovarian malignancy. The diagnosis is usually a surprise made after surgery, based on histopathological findings. Treatment of malignant SO is poorly codified due to the rarity of the tumor [2]. We report two cases of SO, discussing its clinicopathological features, diagnostic criteria and management strategies.
CASE REPORTS
Case 1
A 27-year-old nulligravida patient was referred to our hospital for an incidental ultrasonographic finding of a right-solid adnexal mass. She had no history of ovarian or thyroid disease. Physical examination revealed slight abdominal distension with no palpable pelvic mass or signs of ascites. The serum tumor marker, cancer antigen (CA)-125, was slightly increased at 4 IU/ml (reference value <35 IU/ml). Her pregnancy test was negative. Pelvic ultrasound revealed a right-solid cystic ovarian mass measuring 2.5 × 5.0 × 8.0 cm with intracystic vegetations (Fig. 1).
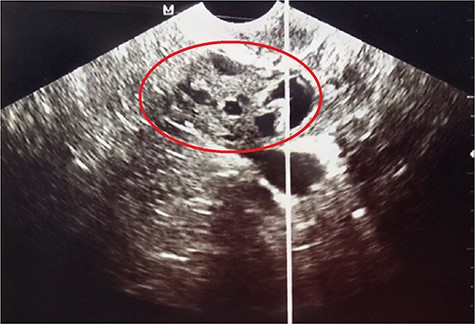
Pelvic ultrasound showing the right-solid cystic ovarian mass with intracystic vegetations (red circle).
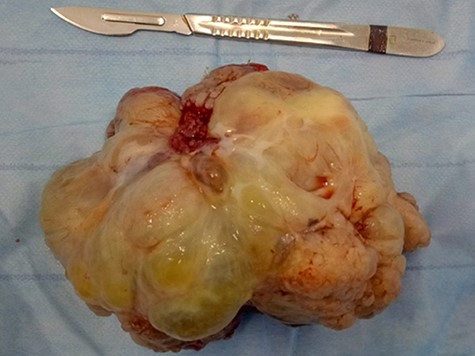
Exploratory laparotomy by a Pfannenstiel incision found a multiloculated complex right- cystic ovarian mass. The left ovary and the rest of the abdomen appeared to be free of disease. Subsequently, a right ovarian cystectomy was performed (Fig. 2). Her postoperative course was uneventful and was discharged on day 2. Histopathological analysis of the surgical specimen revealed a cystic wall with thyroid follicles containing irregular and encapsulated colloid, confirming the diagnosis of a benign SO. Eight months of follow-up with ultrasonography and CA-125 at regular intervals has shown no evidence of recurrence.
Case 2
A 68-year-old postmenopausal multiparous (parity 5) patient with a medical history of hypertension was referred to our hospital for pelvic pains and progressively increasing abdominal mass over 3 months. Physical examination revealed a voluminous abdominopelvic mobile mass with dullness in the flanks. A contrast computerized tomography (CT) scan revealed a large right-solid cystic adnexal tumor measuring 120 × 110 mm with peritoneal carcinosis and left inguinal lymph node metastasis (Fig. 3).
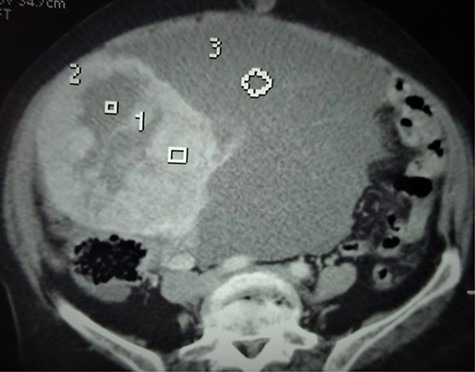
Axial CT scan showing the bulky abdominopelvic mass (3) with solid and cystic components (1 and 2, respectively).
Biological results showed a slight increase in the level of CA-125 at 41 IU/ml. Exploratory laparotomy found a bulky right ovarian mass associated with gelatinous ascites and localized peritoneal carcinosis. The left ovary was free of disease, so a right salpingo-oophorectomy was performed (Fig. 4). She recovered well after surgery and was discharged on day 3. The anatomopathological study revealed a thyroid papillary carcinoma on an ovarian teratoma with multilocular mucinous cystadenoma, consistent with the diagnosis of a malignant SO (Fig. 5A and B).
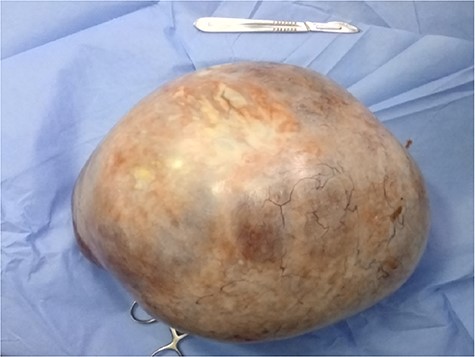
Surgical specimen of the resected bulky right adnexal mass with a thin-wall multilocular cyst.
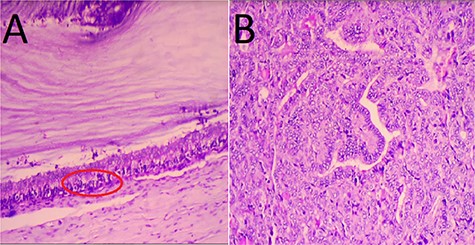
Microscopic findings. (A) Features of thyroid papillary carcinoma on ovarian teratoma with multilocular mucinous cystadenoma (red circle); (B) crowded overlapping nuclei with elongated powdery chromatin. Hematoxylin and eosin staining; magnification: A: ×40, B: ×400.
Additional cervical ultrasound showed a small homogeneous goiter with two small lymph nodes under the right digastric muscle. The patient showed no signs or symptoms of hyperthyroidism, and the hormonal thyroid function test was normal. After discussion in a multidisciplinary team meeting, the choice of therapies was presented and discussed with the patient. She was offered a total abdominal hysterectomy (TAH) with left contralateral salpingo-oophorectomy, lymphadenectomy and total thyroidectomy, followed by 131I radio-ablation, which she refused.
DISCUSSION
SO is a rare tumor accounting for 2.7% of all ovarian teratomas and 1% of all ovarian tumors [2, 3]. It occurs mostly in adults between the ages of 30 and 50 years before menopause. Preoperative clinical and radiologic diagnosis is difficult as the large majority of cases are subclinical. Yoo et al. [3] in their study noted that 41.2% of cases were asymptomatic and tumors were discovered accidentally during routine ultrasound check-up. Clinical symptoms, if present, are mostly nonspecific and include lower abdominal pain and/or a pelvic mass, and less frequently ascites. Despite the majority of thyroid tissue in these tumors, only 5–8% is associated with clinical hyperthyroidism [4].
Laboratory investigations including CA-125 provide little value in diagnosis. The different imaging modalities including ultrasound, CT and magnetic resonance imaging are nonspecific and often suspect of malignancy. Common radiological features include a large complex multilobulated mass with thickened partitions and multiple cysts with solid components. The solid portions take up the contrast product corresponding to the thyroid tissue containing abundant blood vessels and fibrous tissue [2, 5].
A definitive diagnosis is confirmed postoperatively based on histopathological findings of the resected ovary. Gross macroscopic appearance is that of a large solid tumor with a mixed component (mucous and gelatinous content) [2, 6]. A microscopic sample reveals a mature teratoma with thyroid tissue. For a teratoma to be categorized as SO, it must contain >50% thyroid tissue [4]. The pure form is seen as the inclusions of thyroid follicles containing colloid, and the mixed form is associated with other malignant tumors including mucinous cystadenocarcinoma, Brenner tumor, carcinoid tumor and malignant melanoma [4, 7]. The criteria for histopathological diagnosis of malignant SO remain controversial and have varied over the years. The reported rates of malignant transformation range from 5 to 37% [6]. Devaney et al. [6] concluded that the diagnosis of a malignant form is identical to the criteria used for thyroid carcinoma. Malignant SO is classified histopathologically into three types: papillary, follicular and the other variants of carcinoma [4].
Surgical resection remains the main primary treatment. In the case of benign SO, no complete further treatment other than the unilateral oophorectomy is necessary. Conservative surgery (cystectomy or oophorectomy) is recommended [8]. In the case of malignant SO, there is no standard consensus for its management. Many authors agree that due to the many similarities with thyroid cancer, it should be considered and managed as thyroid cancer (ovarian surgery, thyroidectomy and 131I radio-ablation) rather than ovarian cancer (ovarian surgery, radiotherapy and chemotherapy) [1, 2, 7]. The nature of the surgical procedure depends on the locoregional tumor extension and the patient’s age:
Conservative surgery is reserved for younger patients seeking to preserve fertility if the disease is confined to the ovary, followed by definitive surgery after completion of childbearing [7, 9].
Radical surgery, with TAH, bilateral salpingo-oophorectomy, peritoneal lymphadenectomy, thyroidectomy and radio-ablation with 131I should be reserved for more advanced disease and/or in postmenopausal patients [2, 4, 9].
The prognosis of benign struma is very favorable; however, that of malignant struma is poorly evaluated due to the limited number of published cases.
AUTHORS’ CONTRIBUTIONS
All the authors testified to the care of the patient and the writing of the manuscript. The authors have read and agreed with the contents of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
CONSENT
Written informed consent was obtained from the two patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



