-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Graves, James Longoria, Gregory Graves, Cora Ianiro, Leiomyosarcoma of the inferior vena cava: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 11, November 2020, rjaa479, https://doi.org/10.1093/jscr/rjaa479
Close - Share Icon Share
Abstract
Leiomyosarcoma (LMS) of the inferior vena cava (IVC) is an extremely rare malignancy with <400 cases reported. We present a 42-year-old woman with a 3-day history of vague and non-specific abdominal pain. Examination revealed mild tenderness to the epigastrium and right upper quadrant with no other findings. Abdominal ultrasound was performed, which revealed a large hypoechoic mass overlying the IVC. Abdominal computed tomography (CT) was performed which revealed an 8.9 × 7.9 × 9 cm multilobulated lesion encasing the IVC. A CT-guided biopsy was performed which revealed a primary LMS of the IVC. Surgical en bloc excision was performed with an end-to-end Dacron graft for IVC reconstruction. Histopathology confirmed LMS of the vessel wall with negative surgical margins.
INTRODUCTION
Leiomyosarcomas (LMS) are tumors of mesenchymal origin that arise from smooth muscle cells. When they affect vessels, they arise from the tunica media. LMS of the inferior vena cava (IVC) are exceedingly rare, accounting for 0.5% of all adult tissue sarcomas and they affect < 1/100,000 of all adult malignancies [1]. Prognosis is typically poor and definitive treatment is surgical resection with clear margins [2]. The most common symptom of LMS of the IVC is non-specific abdominal pain, but patients can be asymptomatic [3].
In this case report, we present a 42-year-old woman with a 3-day history of non-specific abdominal pain. Ultrasound and (computed tomography) CT supported a diagnosis of an LMS of the IVC with confirmation via CT-guided biopsy.
CASE PRESENTATION
A 42-year-old woman presented to the emergency room with a 3-day history of vague and non-specific abdominal pain. This was the patient’s first time experiencing this type of pain and only presented because her pain was not improving. She reported no other symptoms, no previous medical problems, and no prior surgeries. A pregnancy test was performed and was negative. Examination only revealed mild tenderness in the epigastrium and right upper quadrant with an otherwise a benign exam.
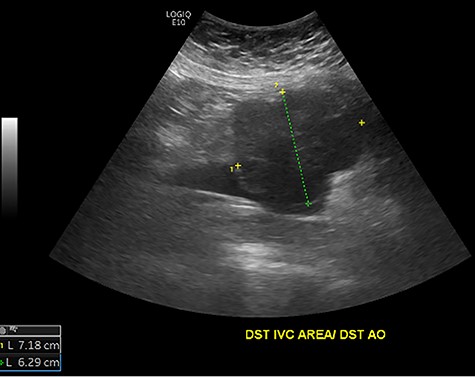
Initial ultrasound showing a large heterogenous, hypoechoic mass like lesion overlying the IVC.
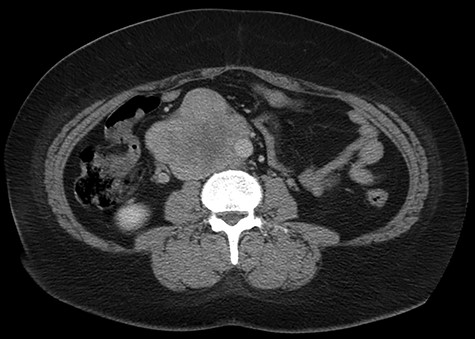
Abdominal CT scan demonstrating IVC lesion that partially encircles the aorta.
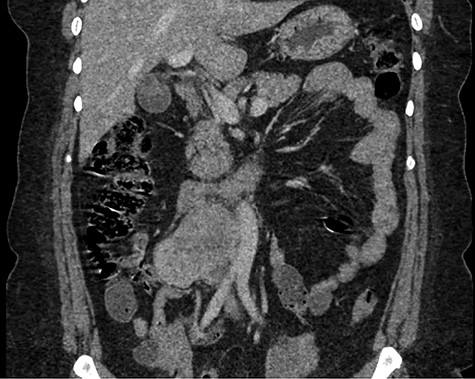
Abdominal CT demonstrating the length and location of the IVC in relation to the aorta.
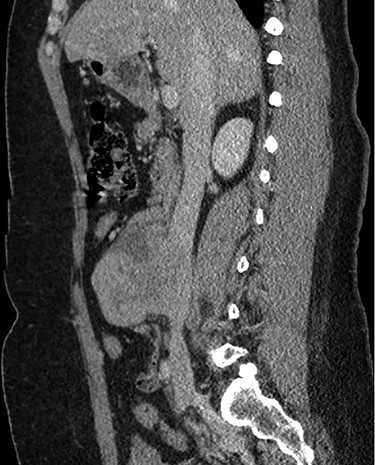
Abdominal CT demonstrating the location of the tumor in the IVC.
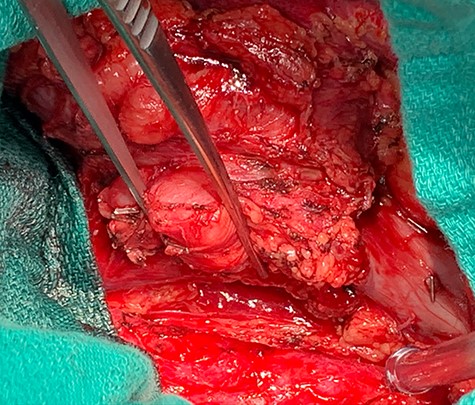
Dissection of primary tumor away abdominal aorta pictured in the lower aspect of the image.
The patient had a body mass index of 40, so combined with her presentation, age and gender. The presumptive diagnosis was of gallbladder pathology, which prompted an abdominal ultrasound. Ultrasound revealed a large 7.7 × 6.3 × 7.2 cm heterogenous, hypoechoic masslike lesion overlying the IVC (Fig 1). Abdominal CT was performed, which revealed an 8.9 × 7.9 × 9 cm multilobulated, heterogeneous soft tissue mass in the right hemiabdomen, encasing the IVC and partially encasing the distal abdominal aorta (Figs. 2–4). CT-guided biopsy was performed, which revealed an LMS.
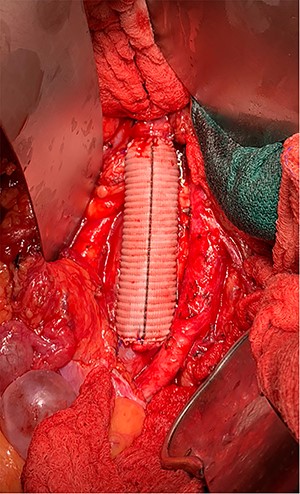
A midline exploratory laparotomy was performed. The tumor extended from inferior to the renal veins to superiorly of the bifurcation. The tumor abutted and partially encased the aorta but did not invade it. Once the tumor was fully mobilized, the patient was heparinized and the vena cava was proximally and distally clamped (Fig 5). The vena cava and tumor were removed en bloc and sent to histopathology. A 22 mm Gelweave Dacron graft was inserted using 5–0 Prolene both proximally and distally (Fig 6). Clamps were removed, and hemostasis was achieved with an overall estimated blood loss of 50 ml. Histopathology of the specimen confirmed an LMS with clear surgical margins.
Patient tolerated the procedure well and had no complications during her hospital stay. Patient was discharged on postoperative Day 4. Patient is scheduled to undergo a CT scan in 3 months for follow-up.
DISCUSSION
LMS are exceedingly rare tumors with < 400 cases reported since their discovery by Perl in 1871 [3]. These tumors are more commonly found in woman (4:1) ratio in the fifth–sixth decade of life [1]. Because of such a rare disease, most literature consists of case studies and case reports, making uniform clinical practice difficult.
Wachtel et al. [3] performed a pooled analysis of 377 patients with LMS and found that median overall survival is reported to be 23 months with overall survival at 1 year at 92 and 55% at 5 years. They found several factors to impact overall survival and disease-free survival with clear surgical margins being one of the most significant.
Two case series directly speak to the impact of clear surgical margins with Hines et al. [4] in a series of 14 patients reported a 5-year survival of 68% in patients with negative margins compared to 0% in patients with positive margins. Hollenbeck et al. [5] in a series of 25 patients reported a similar finding with disease-specific 5-year survival of 33% in patients with negative margins versus 0% in patients with positive margins.
Because of the variable anatomical involvement of the tumor, different surgical resection techniques and approaches should be employed toward specific tumor characteristics and location. Kieffer et al. [6] in a series of 22 patients, divided the IVC into the retro-hepatic or suprarenal, the interrenal and the infrarenal portions as determined by the location of the sarcoma. They reported various methods of access, including the use of bilateral subcostal or midline abdominal incisions. Based on our patient’s tumor location, we utilized a midline laparotomy.
In patients undergoing IVC surgery a surgeon can utilize either a primary repair, patch, or IVC reconstruction as determined by the size and location of the tumor. Because of the importance of clear surgical margins and the size and location of our patient’s tumor, we elected to undergo an IVC reconstruction. Literature surrounding IVC reconstructions have found to be a safe and effective surgical approach to achieve oncologic surgical goals of clear margins while maintaining patient safety. Ruiz et al. [7] in a series of 52 patients for retroperitoneal cancer found a 1-year survival of 75% and 1-year primary patency approaching 90% for patients who underwent IVC construction. We find that due to the importance of clear surgical margins, as previously stated, IVC reconstruction offers the best chance at achieving that goal.
Beyond achieving clear surgical margins, the data regarding the utilization of chemotherapy, radiation therapy, or both in combination with surgical resection is limited, with the best treatment strategy being unclear [8]. In our patient, we elected to forgo utilizing combination therapy, as she had clear surgical margins, and the benefit is not well supported in the literature.
Histopathology from our case was shown to be a well-differentiated LMS with clear surgical margins. It has been well documented that the histology of type retroperitoneal soft tissue sarcoma predicts survival in patients with well-differentiated tumors having better overall survival as compared with poorly differentiated tumors [9].
In conclusion, LMS of the IVC are incredibly rare tumors that will most commonly present in women in their fifth to fifth decade of life. Currently, best treatment is en bloc resection with clear surgical margins. The evidence for the utilization of radiation, chemotherapy or chemoradiation is still in question. For overall success in these complex surgical cases, we suggest a multidisciplinary team of surgeons consisting of surgical oncology and cardiothoracic specialties to address the various oncology and vascular complexities.



