-
PDF
- Split View
-
Views
-
Cite
Cite
Bhawani Khanal, Sunit Agrawal, Roshan Gurung, Suresh Sah, Rakesh Kumar Gupta, Pudendal flap—a good option for creating neo-scrotum after Fournier’s gangrene: a case series, Journal of Surgical Case Reports, Volume 2020, Issue 11, November 2020, rjaa414, https://doi.org/10.1093/jscr/rjaa414
Close - Share Icon Share
Abstract
Scrotal skin loss following Fournier’s gangrene is very distressing to the patients. The management is complex and challenging shown by the multiplicity of flaps and techniques described in the literature.
We included a total of 14 patients with the diagnosis of Fournier’s gangrene over a period of 1 year in our department. We used a modified pudendal thigh flap to reconstruct neo-scrotum in patients with scrotal defects resulting from excision and debridement of Fournier’s gangrene. The average age group of the patients in our study was 41.8 years. The average body mass index in our study was 22.36 kg/m2. The average defect size in our study was 7.05 × 13.07 cm2. There was a single case of flap necrosis. Modified pudendal thigh flap produces a neo-scrotum that looks natural in appearance, provides good quality skin cover and cushion to the testes as well as protective sensation.
INTRODUCTION
Fournier’s gangrene is a necrotizing fasciitis caused by infecting organisms and results in loss of skin and subcutaneous tissue in the perineal area and scrotum [1].
There are many techniques described in the literature including residual scrotal skin mobilization (for defects of scrotal skin up to 50%), split skin graft, thigh flaps with pouch, tissue expansion, gracilis myocutaneous advancement flap, groin fasciocutaneous island flap, flaps based on inferior epigastric vessels, superomedial thigh flaps, pedicled omental flap and even microvascular free greater omentum flap [2]. Factors that determine choice of reconstruction include the size of defect, surgeon’s preference and patient’s choice [3]. The multiplicity of techniques demonstrates that there is potentially no single favored reconstructive technique [3, 4].
The ideal reconstruction would be a single-staged technique that provides soft tissue that protects the denuded testis, maintains thermoregulation to allow spermatogenesis, non-bulky, resilient enough to withstand shearing forces from the thighs and develops a natural looking scrotal ptosis with minimal donor site morbidity [3].
CASE SERIES
Patients presented to our hospital with diagnosis of Fournier’s gangrene from 1 February 2019 to 1 December 2019 were included in this series. Consent for the procedure was taken before the intervention. The detailed clinical and demographic profiles of our patients are presented in the table (Table 1). After extensive debridement patients were admitted to surgical ward for dressing, injectables antibiotics (according to tissue culture sensitivity) and psychiatric counseling. After 2–3 weeks, wounds were evaluated and planned for flap. Patients ready for flap were ensured by healthy granulation tissue over the wound and sterile swab culture sensitivity.
| Cases . | Age (years) . | BMI . | Diagnosis . | Defect area . | Defect size . | Immediate complication . | Late complications . | Comorbidity . |
|---|---|---|---|---|---|---|---|---|
| 1. | 35 | 22.8 | Fournier’s gangrene | b/l testicles | 8 × 15 cm2 | Flap necrosis | None | None |
| 2. | 30 | 18.6 | Fournier’s gangrene | b/l testicles | 7 × 15 cm2 | None | None | None |
| 3. | 38 | 19.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 4. | 43 | 26.3 | Fournier’s gangrene | b/l testicles | 8 × 16 cm2 | None | None | None |
| 5. | 48 | 27.3 | Fournier’s gangrene | b/l testicles | 6 × 12 cm2 | None | None | None |
| 6. | 55 | 32.1 | Fournier’s gangrene | b/l testicles | 7 × 13 cm2 | None | None | Diabetes mellitus |
| 7. | 60 | 23.7 | Fournier’s gangrene | b/l testicles | 6 × 11 cm2 | None | None | Diabetes mellitus |
| 8. | 38 | 21.1 | Fournier’s gangrene | b/l testicles | 8 × 14 cm2 | None | None | None |
| 9. | 40 | 33.6 | Fournier’s gangrene | b/l testicles | 7 × 11 cm2 | None | None | None |
| 10 | 42 | 24.1 | Fournier’s gangrene | b/l testicles | 8 × 11 cm2 | None | None | None |
| 11. | 28 | 21.6 | Fournier’s gangrene | b/l testicles | 6 × 14 cm2 | None | None | None |
| 12. | 52 | 19.7 | Fournier’s gangrene | b/l testicles | 6 × 10 cm2 | None | None | None |
| 13. | 44 | 23.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 14. | 33 | 28.2 | Fournier’s gangrene | b/l testicles | 8 × 13 cm2 | None | None | Diabetes mellitus |
| Cases . | Age (years) . | BMI . | Diagnosis . | Defect area . | Defect size . | Immediate complication . | Late complications . | Comorbidity . |
|---|---|---|---|---|---|---|---|---|
| 1. | 35 | 22.8 | Fournier’s gangrene | b/l testicles | 8 × 15 cm2 | Flap necrosis | None | None |
| 2. | 30 | 18.6 | Fournier’s gangrene | b/l testicles | 7 × 15 cm2 | None | None | None |
| 3. | 38 | 19.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 4. | 43 | 26.3 | Fournier’s gangrene | b/l testicles | 8 × 16 cm2 | None | None | None |
| 5. | 48 | 27.3 | Fournier’s gangrene | b/l testicles | 6 × 12 cm2 | None | None | None |
| 6. | 55 | 32.1 | Fournier’s gangrene | b/l testicles | 7 × 13 cm2 | None | None | Diabetes mellitus |
| 7. | 60 | 23.7 | Fournier’s gangrene | b/l testicles | 6 × 11 cm2 | None | None | Diabetes mellitus |
| 8. | 38 | 21.1 | Fournier’s gangrene | b/l testicles | 8 × 14 cm2 | None | None | None |
| 9. | 40 | 33.6 | Fournier’s gangrene | b/l testicles | 7 × 11 cm2 | None | None | None |
| 10 | 42 | 24.1 | Fournier’s gangrene | b/l testicles | 8 × 11 cm2 | None | None | None |
| 11. | 28 | 21.6 | Fournier’s gangrene | b/l testicles | 6 × 14 cm2 | None | None | None |
| 12. | 52 | 19.7 | Fournier’s gangrene | b/l testicles | 6 × 10 cm2 | None | None | None |
| 13. | 44 | 23.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 14. | 33 | 28.2 | Fournier’s gangrene | b/l testicles | 8 × 13 cm2 | None | None | Diabetes mellitus |
| Cases . | Age (years) . | BMI . | Diagnosis . | Defect area . | Defect size . | Immediate complication . | Late complications . | Comorbidity . |
|---|---|---|---|---|---|---|---|---|
| 1. | 35 | 22.8 | Fournier’s gangrene | b/l testicles | 8 × 15 cm2 | Flap necrosis | None | None |
| 2. | 30 | 18.6 | Fournier’s gangrene | b/l testicles | 7 × 15 cm2 | None | None | None |
| 3. | 38 | 19.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 4. | 43 | 26.3 | Fournier’s gangrene | b/l testicles | 8 × 16 cm2 | None | None | None |
| 5. | 48 | 27.3 | Fournier’s gangrene | b/l testicles | 6 × 12 cm2 | None | None | None |
| 6. | 55 | 32.1 | Fournier’s gangrene | b/l testicles | 7 × 13 cm2 | None | None | Diabetes mellitus |
| 7. | 60 | 23.7 | Fournier’s gangrene | b/l testicles | 6 × 11 cm2 | None | None | Diabetes mellitus |
| 8. | 38 | 21.1 | Fournier’s gangrene | b/l testicles | 8 × 14 cm2 | None | None | None |
| 9. | 40 | 33.6 | Fournier’s gangrene | b/l testicles | 7 × 11 cm2 | None | None | None |
| 10 | 42 | 24.1 | Fournier’s gangrene | b/l testicles | 8 × 11 cm2 | None | None | None |
| 11. | 28 | 21.6 | Fournier’s gangrene | b/l testicles | 6 × 14 cm2 | None | None | None |
| 12. | 52 | 19.7 | Fournier’s gangrene | b/l testicles | 6 × 10 cm2 | None | None | None |
| 13. | 44 | 23.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 14. | 33 | 28.2 | Fournier’s gangrene | b/l testicles | 8 × 13 cm2 | None | None | Diabetes mellitus |
| Cases . | Age (years) . | BMI . | Diagnosis . | Defect area . | Defect size . | Immediate complication . | Late complications . | Comorbidity . |
|---|---|---|---|---|---|---|---|---|
| 1. | 35 | 22.8 | Fournier’s gangrene | b/l testicles | 8 × 15 cm2 | Flap necrosis | None | None |
| 2. | 30 | 18.6 | Fournier’s gangrene | b/l testicles | 7 × 15 cm2 | None | None | None |
| 3. | 38 | 19.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 4. | 43 | 26.3 | Fournier’s gangrene | b/l testicles | 8 × 16 cm2 | None | None | None |
| 5. | 48 | 27.3 | Fournier’s gangrene | b/l testicles | 6 × 12 cm2 | None | None | None |
| 6. | 55 | 32.1 | Fournier’s gangrene | b/l testicles | 7 × 13 cm2 | None | None | Diabetes mellitus |
| 7. | 60 | 23.7 | Fournier’s gangrene | b/l testicles | 6 × 11 cm2 | None | None | Diabetes mellitus |
| 8. | 38 | 21.1 | Fournier’s gangrene | b/l testicles | 8 × 14 cm2 | None | None | None |
| 9. | 40 | 33.6 | Fournier’s gangrene | b/l testicles | 7 × 11 cm2 | None | None | None |
| 10 | 42 | 24.1 | Fournier’s gangrene | b/l testicles | 8 × 11 cm2 | None | None | None |
| 11. | 28 | 21.6 | Fournier’s gangrene | b/l testicles | 6 × 14 cm2 | None | None | None |
| 12. | 52 | 19.7 | Fournier’s gangrene | b/l testicles | 6 × 10 cm2 | None | None | None |
| 13. | 44 | 23.1 | Fournier’s gangrene | b/l testicles | 7 × 14 cm2 | None | None | None |
| 14. | 33 | 28.2 | Fournier’s gangrene | b/l testicles | 8 × 13 cm2 | None | None | Diabetes mellitus |
Our aim was to study immediate complications in terms of wound infection, flap failure and long-term complications in terms of sensation of the skin flap, occurrence of ulceration from shearing against the thighs, walking problems and sexual function.
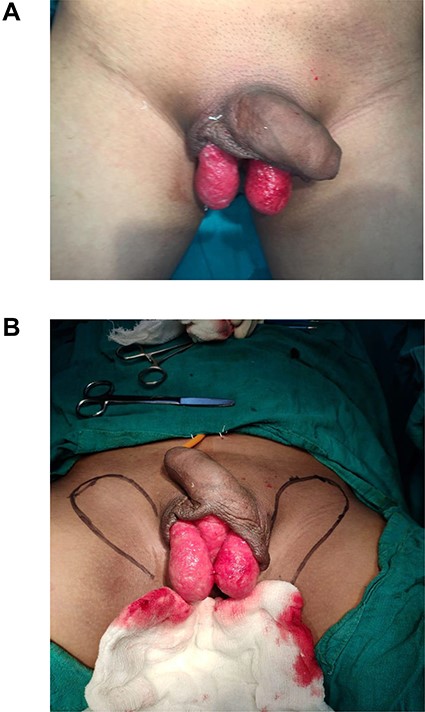
(A) Raw area showing bilateral testis; (B) markings of flaps to be raised.
Techniques
In all cases, a neo-scrotum was created with bilateral pudendal flaps. These flaps were based on the posterior scrotal artery from internal pudendal artery. The perforator of the flap was marked preoperatively with Doppler (Fig. 1B). It was consistently found in the vicinity of the ischial tuberosity.
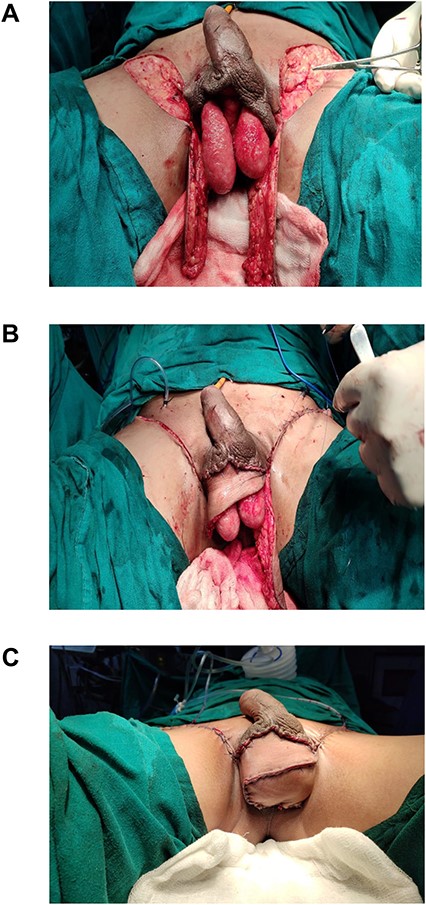
(A) Bilateral pudendal flaps raised; (B) closure of donor area and defect; (C) neo-scrotum.
After spinal anesthesia, patients were kept in lithotomy position. Size of the scrotal defect was calculated using a measuring tape (Fig. 1A). Flaps marking were done as shown in the figure, based on dimension of the defect of scrotum. Assessment of closing the defect directly was checked by a finger pinch test. The flaps were raised bilaterally as a fasciocutaneous flap from distal to proximal along the markings (Fig. 2A). Dissection of the flap was stopped once the length of the flap raised could adequately wrap around the testis—spermatic cord complex. The donor area was closed primarily with Prolene 3-0 CB (Fig. 2B). The flaps were then inset to the defect and secured in place by suturing with Prolene 3-0 CB (Fig. 2C). Two closed suction drains were placed in the donor area in all cases.
Suction drains were removed once the drain output was <30 ml.
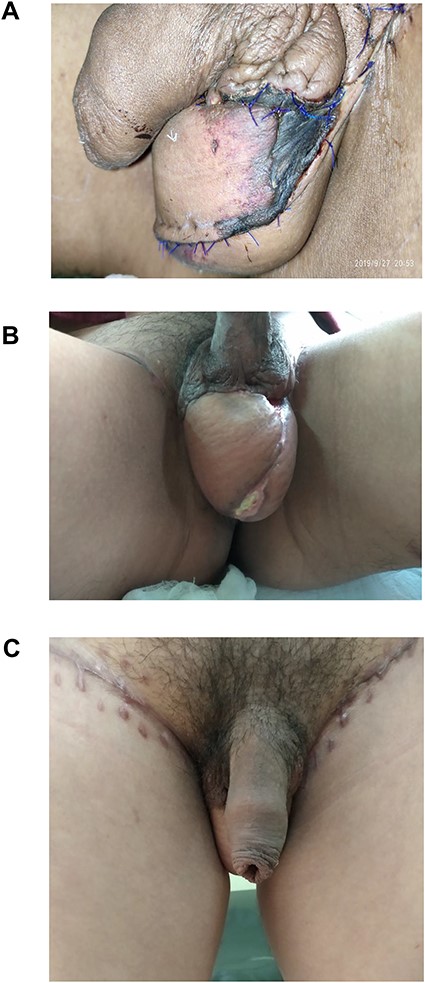
The average age group of the patients in our series was 41.8 years. The average body mass index was 22.36 kg/m2. In our series, there were three patients with diabetes mellitus. The average defect size was 7.05 × 13.07 cm2. Only one case developed flap necrosis towards the edge (Fig. 3A), which was taken care of by daily dressing. Revision surgery included only flap mobilization, which was later without any complications (Fig. 3B and C). There were three married cases with incomplete family size and were in a doubt about their fertility post-procedure, and for whom sperm counts were done after 3 months of the procedure, which came normal.
Early and late complications following the procedure are listed below in tabulated form (Table 1).
DISCUSSION
The affection of genitalia carries special psychological insult as it is a representative sign of masculinity to many patients. The scrotal is elastic and stretchable. When <50% of scrotum is involved, scrotal advancement is a good option. When >50% scrotum is involved, other methods should be considered such as skin grafts, gracilis flap, pudendal flaps and implantation into subcutaneous pouch of thigh.
Burying testis in subcutaneous pouch of thighs bears a lot of problems such as fainting attacks, testicular atrophy, sense of fullness and unacceptable appearances [1]. It also results in disturbances in thermoregulations and adverse psychological effects due to absence of scrotum [5].
Use of local scrotal advancement flap is limited to defects with small size. If used in large defects, it requires additional skin grafts for the complete closure and there have been evidences of flap necrosis [2].
Skin grafts are also described as an option to cover the exposed testis. Minimum requirements for a successful graft include healthy granulation tissue and intact tunica vaginalis. It is also liable for maceration and breakdown [1]. It commonly undergoes contraction and does not provide sufficient protection [5].
The pudendal thigh flap provides good quality skin and good support for already inflamed and denuded testes. The skin and underlying subcutaneous fat provide a good cushioning effect and resilience, which has an advantage over skin grafts. This may be especially helpful for active young patients and keen cyclists [3]. The shape, color and the ‘hang’ or ptosis of the scrotum look ‘natural’ as we had noticed in our patients on long-term follow up. All our patients have expressed psychological satisfaction with the reconstruction. The donor site can be primarily closed and morbidity is minimal.
All patients in our cases have regained some protective sensation and morbidity related to procedure was minimal in our cases. This technique can be used for partial or total reconstruction of scrotum.
Actually, pudendal thigh flap has a good vascular supply and a relatively thin contour. In addition, it is a sensate flap and has close proximity to the scrotum. Moreover, it also leaves a relatively inconspicuous donor site scar because of the proximity with groin creases. In this technique, there is no need of grafts or flaps to close the defect resulting from mobilization of pudendal flap. All these criteria make the pudendal thigh flap superb in comparison with the use of skin grafts or other local flaps. So we used this technique in our cases and the results were excellent.
CONCLUSION
Pudendal flap can be a good option to cover the exposed testis resulting from debridement of Fournier’s gangrene, with good outcomes in terms of natural appearance, good quality skin cover, providing cushion to the testes as well as protective sensation. We recommend further studies with larger sample sizes in order to verify and reinforce this option as a suitable alternative for scrotal reconstruction.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT FROM PATIENT
Patients have provided consent for publication.



