-
PDF
- Split View
-
Views
-
Cite
Cite
Shunichi Ito, Yutaka Takahashi, Takuji Yamada, Yosuke Kawai, Kei Ohira, Intrahepatic cholangiocarcinoma with gastric infiltration misdiagnosed as gastric submucosal tumor, Journal of Surgical Case Reports, Volume 2020, Issue 11, November 2020, rjaa359, https://doi.org/10.1093/jscr/rjaa359
Close - Share Icon Share
Abstract
Intrahepatic cholangiocarcinomas (ICC) are rare primary liver tumors. In few cases, they may invade nearby organs and present as extrahepatic growths, leading to poor prognosis. We report a case of a 78-year-old man who presented with fatigue. An upper gastrointestinal endoscopy was performed to find a cause for his anemia, which showed a submucosal tumor with delle at the lesser curvature of the gastric cardia. A computed tomography revealed a low-density tumor of diameter 70 mm at the cardia. It appeared to infiltrate the liver directly. We performed lateral hepatectomy, proximal gastrectomy and lymphadenectomy. The pathological findings revealed an ICC with gastric infiltration. Although adjuvant chemotherapy was administered, 12 months postoperatively, the patient developed pain and multiple bone metastases for which palliative radiation was initiated. The guidelines for lymphadenectomy and adjuvant chemotherapy in such cases are unclear. Appropriate regional lymphadenectomy and adjuvant chemotherapy can improve the prognosis of such patients.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is a rare primary liver tumor, and accounts for 4.8% of all primary liver cancers in Japan [1]. Occasionally, an ICC presents as an extrahepatic growth. In few cases, ICC may invade nearby organs. Herein, we present a case of ICC with gastric infiltration that appeared like a submucosal tumor (SMT).
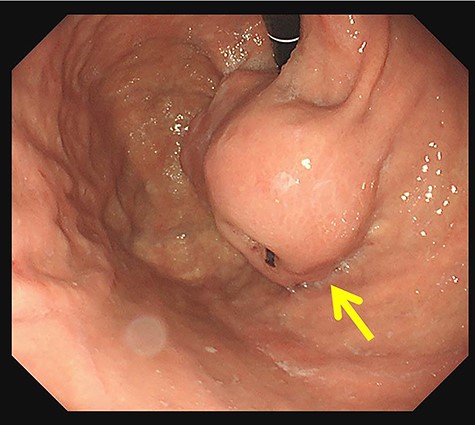
In upper gastrointestinal endoscopy, the tumor looked like a submucosal tumor with delle at the lesser curvature of the cardia (arrow).
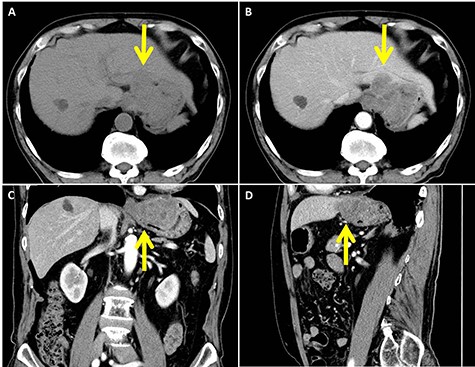
In abdominal enhanced computed tomography, a slightly low-density tumor of about 70 mm in diameter at the cardia (arrow) was seen. It appeared to infiltrate into the liver directly. (A: plane; B: axial image in the artery phase; C: coronal image in the artery phase; D: sagittal image in the artery phase)
CASE REPORT
A 78-year-old man visited a hospital for fatigue. He underwent an upper gastrointestinal (GI) endoscopy for anemia, and was diagnosed with a gastric SMT. He was referred to our hospital for further treatment. His family history was unremarkable. His past history included atrial fibrillation, right cerebral infarction and hypertension. His physical examination was unremarkable.
On investigating, his hemoglobin was 10 g/dl, and tumor markers, carcinoembryonic antigen and carbohydrate antigen 19-9 (CA 19-9), were elevated to 12.5 ng/ml and 94.3 U/ml, respectively. Tests for Hepatitis B and C viruses were negative. His upper GI endoscopy showed SMT with delle at the lesser curvature of the cardia (Fig. 1). A computed tomography (CT) showed a slightly low-density tumor of 70 mm in diameter at the cardia (Fig. 2). It appeared to infiltrate into the liver directly. We diagnosed it as gastric SMT (suspected GI stromal tumor) with liver infiltration.
The patient was taken up for surgery. Intraoperatively, the tumor was located in the stomach wall at the cardia and was invading the liver directly (Fig. 3). We performed a lateral hepatectomy by the Glissonean pedicle ligation method, proximal gastrectomy and lymphadenectomy. The operation time was 3 h and 19 min, and intraoperative blood loss was 260 ml. The resected specimen showed a white solid tumor of dimensions 80 mm × 55 mm × 44 mm. It showed a little ulcerative change in the mucosal layer (Fig. 4). Most of the tumor originating from the liver was found under the gastric mucosa (Figs 4 and 5). Microscopically, a poorly differentiated adenocarcinoma with unclear duct formation was detected (Fig. 6). The non-tumor area of the liver was normal. Immunostaining showed that the tumor was positive for AE1/AE3 and negative for CK20, CK7, CD34, c-kit, CD56, synaptophysin and chromogranin A (Fig. 6). Based on these findings, our diagnosis was ICC with gastric infiltration, and we categorized it as pT4, pN0, cM0, Stage IIIB as per the eighth edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) [2]. The immediate postoperative course was uneventful, and the patient was discharged on postoperative Day 16. Three months after the surgery, he was administered S-1 orally as adjuvant chemotherapy. However, 12 months after the surgery, multiple bone metastases were detected on CT. He underwent palliative radiation to relieve pain and prevent pathological fractures.
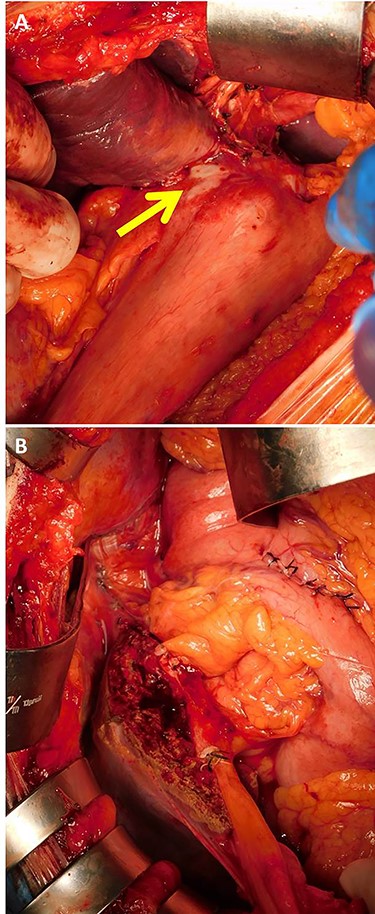
Intraoperative findings showed that the tumor was located in the stomach wall of the cardia and was invading into the liver directly (A, arrow). Lateral hepatectomy, proximal gastrectomy, and lymphadenectomy were performed (B).
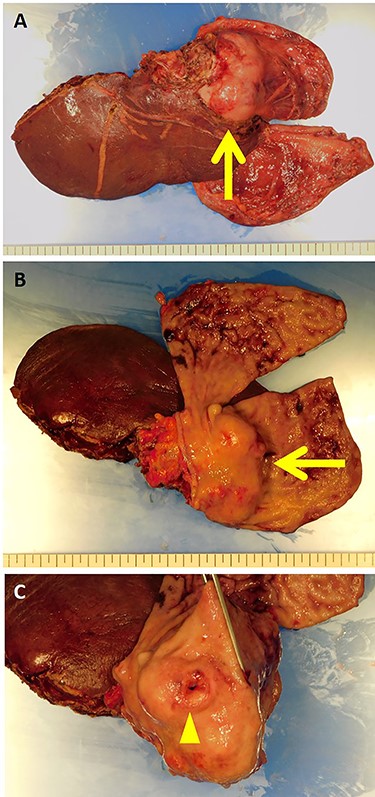
The resected specimen showed a white solid tumor continued from the left lateral liver to the stomach wall (A, arrow). Most of the tumor was found under the gastric mucosa (B, arrow). The tumor looked like a submucosal tumor with delle (C, arrowhead).
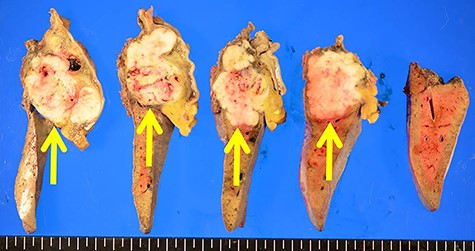
The tumor originated from the liver and showed extrahepatic growth (arrow).
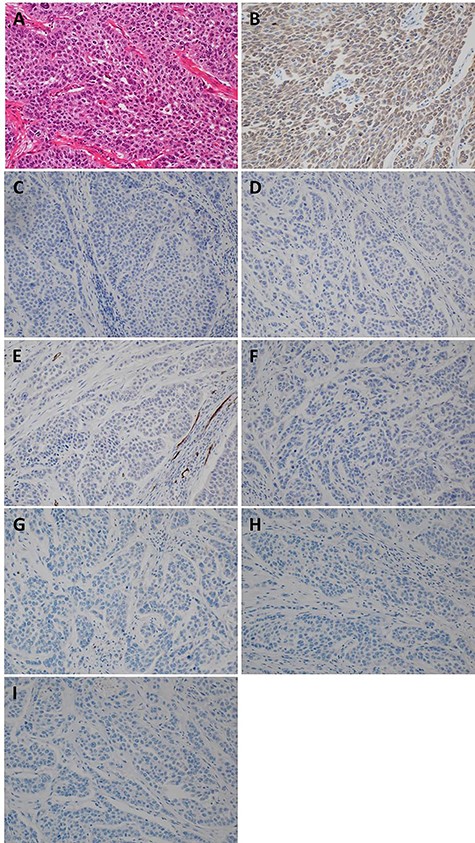
Pathological and immunostaining findings. (A) Hematoxylin–Eosin staining (×20): A poorly differentiated adenocarcinoma with unclear duct formation was detected. (B) AE1/AE3 staining (×20): positive. (C) CK20 staining (×20): negative. (D) CK7 staining (×20): negative. (E) CD34 staining (×20): negative. (F) c-kit staining (×20): negative. (G) CD56 staining (×20): negative. (H) synaptophysin staining (×20): negative. (I) chromogranin A staining (×20): negative.
DISCUSSION
ICC has a poor prognosis and an overall 5-year survival of <10% for all patients [3]. Unfortunately, 80–85% of patients with ICC present at an unresectable stage of the disease, with no potential for cure [4]. Kang et al. [5] indicated in a multivariate analysis that independent risk factors for tumor recurrence and patient survival were multiple tumors; CA, 19-9 > 200 U/mL; tumor size, > 5 cm; direct invasion to extrahepatic structures; and lymph node metastases. Although a surgery, such as hepatectomy, is considered the first option of treatment for ICC, the role of lymph node excision is still unclear, with no clear guidelines [6]. Giorgio et al. [6] report that routine regional lymphadenectomy is the most recent therapeutic strategy for ICC because the incidence of lymph node metastases is 40% for ICC. Nozaki et al. [7] suggested that regional lymphadenectomy should be performed in consideration with tumor location and the two main lymphatic drainage routes: one through the hepatoduodenal ligament and other through the cardiac portion of the stomach. If the tumor is located in the right lobe, lymphadenectomy in the former route may be necessary, and if it is located in the left lobe such as in our case, lymphadenectomy in both routes may be necessary. However, since our case was misdiagnosed as gastric SMT, we performed lymphadenectomy only in one route. Bartsch et al. [8] studied 102 cases of hepatectomy for ICC in a single center. Out of these 102, six had visceral infiltration—two in the diaphragm, one in adrenal gland, one in pericardium, one in duodenum and one in colon. They also reported that patients with visceral infiltration had a significantly shorter overall survival than patients without it (14.9 vs. 23.1 months) [8].
Clinicopathological features of resected intrahepatic cholangiocarcinoma with visceral infiltration reported in the literature
| Operative procedure . | LD . | Visceral infiltration . | Pathological diagnosis . | Tumor size (cm) . | CA19–9 (U/ml) . | Adjuvant chemotherapy . | Survival after surgery . |
|---|---|---|---|---|---|---|---|
| Left hepatectomy, Caudate lobectomy Total gastrectomy, Partial diaphragmectomy | + | Stomach | Well differentiated AC | 5.5 × 5 | 7510 | + Tegafur | Not described |
| Left hepatectomy Hemigastrectomy | + | Stomach | AC with sarcomatous elements | 7.7 | WNL | ND | Dead due to metastases 5 months |
| Right hepatectomy, Right adrenalectomy Caval resection and reconstruction | ND | Right AG | Moderately differentiated AC | 6.5 × 5.5 | WNL | ND | Alive without recurrence 22 months |
| Right hepatectomy Partial diaphragmectomy | − | Diaphragm | Poorly differentiated AC | 7 × 6 × 3 | 23 | ND | Alive without recurrence 24 months |
| Left hepatectomy, Caudate lobectomy Partial gastrectomy | + | Stomach | Moderately differentiated AC | 6.4 × 5.8 × 5 | 17 | ND | ND |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | Adenosquamous cell carcinoma | 11 × 8 | ND | ND | Dead due to metastasis 6 months |
| Right hepatic trisegmentectomy | + | Gallbladder | Well differentiated AC | 11 × 9 × 6 | >12000 | ND | Alive without recurrence 7 months |
| Left hepatectomy, Caudate lobectomy Distal gastrectomy | + | Stomach | Moderately differentiated AC | 5 | 151.7 | + GEM | Alive without recurrence 18 months |
| Right hepatectomy Right hemicolectomy | + | TC | Poorly differentiated AC | 12 × 10 | WNL | + Tegaful-urasil | Alive after treatment for brain metastasis 84 months |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | AC | 9 × 6.5 × 6 | 1518 | ND | Alive with recurrence 3 months |
| Right hepatectomy, Partial diaphragmectomy Right adrenalectomy, Right nephrectomy | ND | Diaphragm, Right AG Right kidney | Poorly differentiated AC | 9 × 7.2 | 15.9 | ND | Dead due to recurrence 6 months |
| Extended right hepatectomy, Caudate lobectomy Partial diaphragmectomy, Right adrenalectomy | + | Right AG | Moderately differentiated AC | 10 | 222 | + GEM | Alive without recurrence 12 months |
| Lateral hepatectomy, Total gastrectomy Partial pancreatectomy | + | Stomach Pancreas | Moderately differentiated AC | 9 | 9899 | − | Alive with recurrence 8 months |
| Central bisegmentectomy Partial diaphragmectomy | ND | Diaphragm | Poorly differentiated AC | 4 | WNL | ND | Alive after treatment for diaphragm recurrence 29 months |
| Partial resection of the S4 Partial duodenectomy | + | Duodenum | Moderately differentiated AC | 3.3 | WNL | + GEM | Alive after treatment for liver recurrence 21 months |
| Subsegmentectomy of the S4a and S5 Partial colectomy | + | TC Gallbladder | Moderately differentiated AC | 7 × 4.8 × 4 | 3605.5 | + GEM | Alive without recurrence 9 months |
| Extended cholecystectomy, Partial gastrectomy Partial duodenectomy, Partial colectomy | + | Stomach, Duodenum TC, Gallbladder | Poorly differentiated AC | 9 | 38 | + GEM/Cisplatin | Dead due to recurrence 9 months |
| Lateral hepatectomy Proximal gastrectomy | + | Stomach | Poorly differentiated AC | 8 × 5.5 × 4.4 | 94.3 | + S-1 | Alive with recurrence 12 months |
| Operative procedure . | LD . | Visceral infiltration . | Pathological diagnosis . | Tumor size (cm) . | CA19–9 (U/ml) . | Adjuvant chemotherapy . | Survival after surgery . |
|---|---|---|---|---|---|---|---|
| Left hepatectomy, Caudate lobectomy Total gastrectomy, Partial diaphragmectomy | + | Stomach | Well differentiated AC | 5.5 × 5 | 7510 | + Tegafur | Not described |
| Left hepatectomy Hemigastrectomy | + | Stomach | AC with sarcomatous elements | 7.7 | WNL | ND | Dead due to metastases 5 months |
| Right hepatectomy, Right adrenalectomy Caval resection and reconstruction | ND | Right AG | Moderately differentiated AC | 6.5 × 5.5 | WNL | ND | Alive without recurrence 22 months |
| Right hepatectomy Partial diaphragmectomy | − | Diaphragm | Poorly differentiated AC | 7 × 6 × 3 | 23 | ND | Alive without recurrence 24 months |
| Left hepatectomy, Caudate lobectomy Partial gastrectomy | + | Stomach | Moderately differentiated AC | 6.4 × 5.8 × 5 | 17 | ND | ND |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | Adenosquamous cell carcinoma | 11 × 8 | ND | ND | Dead due to metastasis 6 months |
| Right hepatic trisegmentectomy | + | Gallbladder | Well differentiated AC | 11 × 9 × 6 | >12000 | ND | Alive without recurrence 7 months |
| Left hepatectomy, Caudate lobectomy Distal gastrectomy | + | Stomach | Moderately differentiated AC | 5 | 151.7 | + GEM | Alive without recurrence 18 months |
| Right hepatectomy Right hemicolectomy | + | TC | Poorly differentiated AC | 12 × 10 | WNL | + Tegaful-urasil | Alive after treatment for brain metastasis 84 months |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | AC | 9 × 6.5 × 6 | 1518 | ND | Alive with recurrence 3 months |
| Right hepatectomy, Partial diaphragmectomy Right adrenalectomy, Right nephrectomy | ND | Diaphragm, Right AG Right kidney | Poorly differentiated AC | 9 × 7.2 | 15.9 | ND | Dead due to recurrence 6 months |
| Extended right hepatectomy, Caudate lobectomy Partial diaphragmectomy, Right adrenalectomy | + | Right AG | Moderately differentiated AC | 10 | 222 | + GEM | Alive without recurrence 12 months |
| Lateral hepatectomy, Total gastrectomy Partial pancreatectomy | + | Stomach Pancreas | Moderately differentiated AC | 9 | 9899 | − | Alive with recurrence 8 months |
| Central bisegmentectomy Partial diaphragmectomy | ND | Diaphragm | Poorly differentiated AC | 4 | WNL | ND | Alive after treatment for diaphragm recurrence 29 months |
| Partial resection of the S4 Partial duodenectomy | + | Duodenum | Moderately differentiated AC | 3.3 | WNL | + GEM | Alive after treatment for liver recurrence 21 months |
| Subsegmentectomy of the S4a and S5 Partial colectomy | + | TC Gallbladder | Moderately differentiated AC | 7 × 4.8 × 4 | 3605.5 | + GEM | Alive without recurrence 9 months |
| Extended cholecystectomy, Partial gastrectomy Partial duodenectomy, Partial colectomy | + | Stomach, Duodenum TC, Gallbladder | Poorly differentiated AC | 9 | 38 | + GEM/Cisplatin | Dead due to recurrence 9 months |
| Lateral hepatectomy Proximal gastrectomy | + | Stomach | Poorly differentiated AC | 8 × 5.5 × 4.4 | 94.3 | + S-1 | Alive with recurrence 12 months |
AC: adenocarcinoma, AG: adrenal grand, GEM: gemcitabine, LD: lymph node dissection, ND: not described, RFA: radiofrequency ablation, TACE: transcatheter arterial chemoembolization, TC: transverse colon, WNL: within normal limit
Clinicopathological features of resected intrahepatic cholangiocarcinoma with visceral infiltration reported in the literature
| Operative procedure . | LD . | Visceral infiltration . | Pathological diagnosis . | Tumor size (cm) . | CA19–9 (U/ml) . | Adjuvant chemotherapy . | Survival after surgery . |
|---|---|---|---|---|---|---|---|
| Left hepatectomy, Caudate lobectomy Total gastrectomy, Partial diaphragmectomy | + | Stomach | Well differentiated AC | 5.5 × 5 | 7510 | + Tegafur | Not described |
| Left hepatectomy Hemigastrectomy | + | Stomach | AC with sarcomatous elements | 7.7 | WNL | ND | Dead due to metastases 5 months |
| Right hepatectomy, Right adrenalectomy Caval resection and reconstruction | ND | Right AG | Moderately differentiated AC | 6.5 × 5.5 | WNL | ND | Alive without recurrence 22 months |
| Right hepatectomy Partial diaphragmectomy | − | Diaphragm | Poorly differentiated AC | 7 × 6 × 3 | 23 | ND | Alive without recurrence 24 months |
| Left hepatectomy, Caudate lobectomy Partial gastrectomy | + | Stomach | Moderately differentiated AC | 6.4 × 5.8 × 5 | 17 | ND | ND |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | Adenosquamous cell carcinoma | 11 × 8 | ND | ND | Dead due to metastasis 6 months |
| Right hepatic trisegmentectomy | + | Gallbladder | Well differentiated AC | 11 × 9 × 6 | >12000 | ND | Alive without recurrence 7 months |
| Left hepatectomy, Caudate lobectomy Distal gastrectomy | + | Stomach | Moderately differentiated AC | 5 | 151.7 | + GEM | Alive without recurrence 18 months |
| Right hepatectomy Right hemicolectomy | + | TC | Poorly differentiated AC | 12 × 10 | WNL | + Tegaful-urasil | Alive after treatment for brain metastasis 84 months |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | AC | 9 × 6.5 × 6 | 1518 | ND | Alive with recurrence 3 months |
| Right hepatectomy, Partial diaphragmectomy Right adrenalectomy, Right nephrectomy | ND | Diaphragm, Right AG Right kidney | Poorly differentiated AC | 9 × 7.2 | 15.9 | ND | Dead due to recurrence 6 months |
| Extended right hepatectomy, Caudate lobectomy Partial diaphragmectomy, Right adrenalectomy | + | Right AG | Moderately differentiated AC | 10 | 222 | + GEM | Alive without recurrence 12 months |
| Lateral hepatectomy, Total gastrectomy Partial pancreatectomy | + | Stomach Pancreas | Moderately differentiated AC | 9 | 9899 | − | Alive with recurrence 8 months |
| Central bisegmentectomy Partial diaphragmectomy | ND | Diaphragm | Poorly differentiated AC | 4 | WNL | ND | Alive after treatment for diaphragm recurrence 29 months |
| Partial resection of the S4 Partial duodenectomy | + | Duodenum | Moderately differentiated AC | 3.3 | WNL | + GEM | Alive after treatment for liver recurrence 21 months |
| Subsegmentectomy of the S4a and S5 Partial colectomy | + | TC Gallbladder | Moderately differentiated AC | 7 × 4.8 × 4 | 3605.5 | + GEM | Alive without recurrence 9 months |
| Extended cholecystectomy, Partial gastrectomy Partial duodenectomy, Partial colectomy | + | Stomach, Duodenum TC, Gallbladder | Poorly differentiated AC | 9 | 38 | + GEM/Cisplatin | Dead due to recurrence 9 months |
| Lateral hepatectomy Proximal gastrectomy | + | Stomach | Poorly differentiated AC | 8 × 5.5 × 4.4 | 94.3 | + S-1 | Alive with recurrence 12 months |
| Operative procedure . | LD . | Visceral infiltration . | Pathological diagnosis . | Tumor size (cm) . | CA19–9 (U/ml) . | Adjuvant chemotherapy . | Survival after surgery . |
|---|---|---|---|---|---|---|---|
| Left hepatectomy, Caudate lobectomy Total gastrectomy, Partial diaphragmectomy | + | Stomach | Well differentiated AC | 5.5 × 5 | 7510 | + Tegafur | Not described |
| Left hepatectomy Hemigastrectomy | + | Stomach | AC with sarcomatous elements | 7.7 | WNL | ND | Dead due to metastases 5 months |
| Right hepatectomy, Right adrenalectomy Caval resection and reconstruction | ND | Right AG | Moderately differentiated AC | 6.5 × 5.5 | WNL | ND | Alive without recurrence 22 months |
| Right hepatectomy Partial diaphragmectomy | − | Diaphragm | Poorly differentiated AC | 7 × 6 × 3 | 23 | ND | Alive without recurrence 24 months |
| Left hepatectomy, Caudate lobectomy Partial gastrectomy | + | Stomach | Moderately differentiated AC | 6.4 × 5.8 × 5 | 17 | ND | ND |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | Adenosquamous cell carcinoma | 11 × 8 | ND | ND | Dead due to metastasis 6 months |
| Right hepatic trisegmentectomy | + | Gallbladder | Well differentiated AC | 11 × 9 × 6 | >12000 | ND | Alive without recurrence 7 months |
| Left hepatectomy, Caudate lobectomy Distal gastrectomy | + | Stomach | Moderately differentiated AC | 5 | 151.7 | + GEM | Alive without recurrence 18 months |
| Right hepatectomy Right hemicolectomy | + | TC | Poorly differentiated AC | 12 × 10 | WNL | + Tegaful-urasil | Alive after treatment for brain metastasis 84 months |
| Right hepatectomy, Partial diaphragmectomy Right lower partial pneumonectomy | ND | Lung Diaphragm | AC | 9 × 6.5 × 6 | 1518 | ND | Alive with recurrence 3 months |
| Right hepatectomy, Partial diaphragmectomy Right adrenalectomy, Right nephrectomy | ND | Diaphragm, Right AG Right kidney | Poorly differentiated AC | 9 × 7.2 | 15.9 | ND | Dead due to recurrence 6 months |
| Extended right hepatectomy, Caudate lobectomy Partial diaphragmectomy, Right adrenalectomy | + | Right AG | Moderately differentiated AC | 10 | 222 | + GEM | Alive without recurrence 12 months |
| Lateral hepatectomy, Total gastrectomy Partial pancreatectomy | + | Stomach Pancreas | Moderately differentiated AC | 9 | 9899 | − | Alive with recurrence 8 months |
| Central bisegmentectomy Partial diaphragmectomy | ND | Diaphragm | Poorly differentiated AC | 4 | WNL | ND | Alive after treatment for diaphragm recurrence 29 months |
| Partial resection of the S4 Partial duodenectomy | + | Duodenum | Moderately differentiated AC | 3.3 | WNL | + GEM | Alive after treatment for liver recurrence 21 months |
| Subsegmentectomy of the S4a and S5 Partial colectomy | + | TC Gallbladder | Moderately differentiated AC | 7 × 4.8 × 4 | 3605.5 | + GEM | Alive without recurrence 9 months |
| Extended cholecystectomy, Partial gastrectomy Partial duodenectomy, Partial colectomy | + | Stomach, Duodenum TC, Gallbladder | Poorly differentiated AC | 9 | 38 | + GEM/Cisplatin | Dead due to recurrence 9 months |
| Lateral hepatectomy Proximal gastrectomy | + | Stomach | Poorly differentiated AC | 8 × 5.5 × 4.4 | 94.3 | + S-1 | Alive with recurrence 12 months |
AC: adenocarcinoma, AG: adrenal grand, GEM: gemcitabine, LD: lymph node dissection, ND: not described, RFA: radiofrequency ablation, TACE: transcatheter arterial chemoembolization, TC: transverse colon, WNL: within normal limit
In the eighth edition of the AJCC/UICC, T4 is defined as ‘tumor involving local extrahepatic structures by direct invasion’ [2]. To the best of our knowledge, resected ICCs with visceral infiltration have been reported only in three cases in English literature and in 14 cases in Japanese literature (Table 1). The median age of these patients was 63.5 years (range: 41–85). The median size of the tumor was 7.9 cm (range: 3.3–12), and except for one case, the size of the tumor was larger than 5 cm (94%). In six cases, CA 19-9 was over 200 U/L. In eight cases, adjuvant chemotherapy was administered—four, used gemcitabine; one, gemcitabine and cisplatin; one, tegafur; one, tegaful-urasil; and one, S-1. Visceral infiltration was noted for seven in the diaphragm; five, stomach; three, duodenum; three, transverse colon; three, gallbladder; three, lung; two, right adrenal gland; one, right kidney; and one, pancreas (some patients had visceral infiltration in more than one organ). Among the five cases of stomach infiltration, two showed SMT with delle on upper GI endoscopy. Lymphadenectomy was performed in 11 cases (61%). Within 6 months after surgery, three patients died from recurrence.
Like for lymph node excision, no clear guidelines exist for administration of adjuvant chemotherapy for such cases. However, adjuvant chemotherapy with Capecitabine can improve the overall survival in patients with resected biliary tract cancer [3].
Only a few cases of resected ICCs with visceral infiltration have been reported in the literature. Therefore, we believe, our case report can help to understand the characteristic features of these rare cases and, as a result, improve their prognosis.
CONFLICTS OF INTEREST STATEMENT
None declared.
References
- anemia
- computed tomography
- bone metastasis
- cholangiocarcinoma
- adjuvant chemotherapy
- upper gastrointestinal endoscopy
- fatigue
- liver neoplasms
- hepatic resection
- lymph node excision
- pain
- cardia
- growth
- guidelines
- liver
- neoplasms
- palliative care
- lymph node dissection
- cholangiocarcinoma, intrahepatic
- proximal subtotal gastrectomy
- misdiagnosis
- infiltrates
- regional lymph node dissection



