-
PDF
- Split View
-
Views
-
Cite
Cite
Goran Gudelj, Tena Simunjak, Marica Zizic, Boris Simunjak, Martin Jurlina, An unusual case of hyalinizing clear cell carcinoma in a sinonasal cavity and cranial base, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa436, https://doi.org/10.1093/jscr/rjaa436
Close - Share Icon Share
Abstract
Hyalinizing clear cell carcinoma (HCCC) is a rare, predominantly minor salivary gland tumor. Most of these tumors occur in the oral cavity, mainly the palate and tongue. Primary localization of the tumor in the region of the nasal cavity and paranasal sinuses is extremely rare and, with only a few cases reported in the literature so far. We present an extremely rare case of a 61-year-old woman with hyalinizing clear cell carcinoma (HCCC), occupying the left posterior nasal and nasopharyngeal cavity, as a primary tumor location. The patient total recovery was uneventful, and she is now free of disease at three years postoperative follow-up.
INTRODUCTION
Hyalinizing clear cell carcinoma (HCCC) is a rare malignant salivary gland tumor of epithelial origin, characterized by nests and cords of clear cells surrounded by hyalinizing stroma. It is in more than 80% of cases located in minor salivary glands of the oral and oropharyngeal cavity. Nasal cavity and paranasal sinus are rarely affected with only a few cases published so far [1,2]. In terms of epidemiology, it is a rare entity with an incidence less than 1% of all salivary gland tumors. It appears in the 5–6th decade, with a slight female predominance.
CASE REPORT
A 61-year-old female patient presented with history of disease lasted for 5 years before hospital admission. Symptoms included nasal obstruction with consecutive conductive hearing loss, hyposmia, nasal discharge and nasality in speech. Nasal endoscopic examination showed a tumor mass in the posterior part of the left nasal cavity occupying both sides of the nasopharyngeal space. Multi-slice CT scan and MRI revealed a destructive tumor mass 4 × 5 cm primary located in the region of paranasal sinuses on the left side with suspected tumor spread in the nasopharynx, upper parapharyngeal space and petroclival region with rather extensive skull base involvement, as it is demonstrated in Fig. 1.
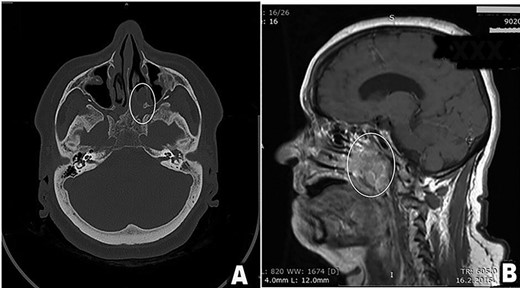
(A and B) Preoperative scan (A-axial CT scan, B-sagittal MRI scan). Multi-sliced CT and MRI scan revealed a destructive mass 4 × 5 cm in the posterior third of the left nasal cavity, left ethmoid and left maxillary sinus spreading into nasopharynx predominantly on patients left side, but occluding both choanae (tumor in white oval). Tumor spread included left great wing of the sphenoid bone, along the left petroclival fissure and the apex of the left temporal bone.
The endoscopic endonasal approach under neuronavigation control was preferred surgical technique, broadened with Caldwell-Luc procedure through the left maxillary sinus. The left common carotid artery was presented in the neck to enable its ligation in the case of unexpected intraoperative damage to the left internal carotid artery (ICA). The surgical procedure began with a Caldwell-Luc procedure on the patient’s left side, followed with inferior medial maxillectomy after Kamel [3]. Hence, the formation of a large unified space of the nasal cavity and maxillary sinus on the patient’s left side has enabled us to securely work far lateral in the zone of the infratemporal fossa. The next step was drilling of the posterior maxillary sinus wall with presentation and ligation of the pterygopalatine artery. Pterygoid plates were then completely drilled out and cartilage portion of the Eustachian tube exposed and transected, along with the resection of the structures of pterygopalatine and pterygomaxillary spaces. The tumor was liberated from the surrounding tissues and completely resected from the left parapharyngeal space. Cranially, it was followed with the resection in the zone of Meckel’s cave, where a cuff of the tumor around the ICA was left behind because it was surrounded in circumference over 180°. We have managed to achieve preoperatively planned tumor resection. Taking into account preoperative histopathologic diagnosis, a tumor remnant was left behind in contact with the left ICA, since it was impossible to reach complete excision without endangering its integrity. There were no intraoperative complications, and postoperative recovery was uneventful. Histopathologic description revealed a tumor tissue composed of nests of the tumor cells that have clear cell cytoplasm. After first histopathologic analysis based on hematoxylin and eosin staining, differential diagnosis included: tumor-like sinonasal renal cell-like adenocarcinoma (SRCLA) and HCCC, which may be difficult to differentiate between each other. Immunohistochemistry showed positive staining of cells on CK7, AE1/AE3, Vimentin and focal positivity on EMA antigens. Cell staining was negative on HMB45, SMA, CD10 and S100 antigens. After additional nephrological examination, PET scan and CT scan, nephrological etiology and the possibility of metastatic SRCLA were excluded. The final diagnosis of HCCC was established.
In the light of unexpected histopathological diagnosis with residual tumor, the decision to apply adjuvant radiotherapy was made. The total tumor dose of 70 Gy was applied during 8 weeks in 35 fractions in a form of 3D conformal adjuvant radiotherapy.
Till this day, the patient is free of local tumor recurrence and distant tumor as illustrated in Fig. 2A–C.
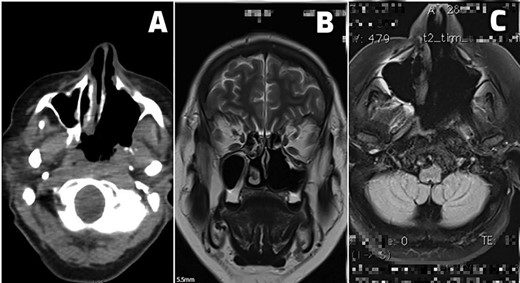
(A–C) Postoperative scans. (A) An early postoperative PET CT scan shows tumor margins and postoperative sinonasal cavity. (B and C) MRI scans of patients free of local tumor recurrence and distant tumor spread at 24 months (B) and 36 months (C) of regular postoperative follow-up.
DISCUSSION
In 1988, Peison and colleagues reported one case of nasal clear cell mucoepidermoid carcinoma, which was first described in detail and named by Milchgrub et al. as HCCC [4,5]. HCCC is a malignant neoplasm of the salivary gland origin, most often occurring between the 4th and 7th decade of life. Small salivary glands are the most common primary site of HCCC, with the palate reported as the most frequently affected, followed in descending order by the buccal mucosa, the base of the tongue, the floor of the mouth, lips, retromolar and the tonsillar region. It seldom occurs in large salivary glands. Swelling is often the only sign of a tumor, but ulceration and pain may also be present. HCCC extremely rare occurs in the nasal cavity and paranasal sinuses. In most of the reports, patients leading symptoms are related to the tumor volume, resulting in nose obstruction and secretion that often confines to epistaxis, anosmia, as well as the symptoms of Eustachian tube closure and consequent conductive hearing impairment [6]. The reported duration of symptoms varies from one month to 15 years. The slow and indolent growth of the tumor is one of its most important features [7]. Despite its size, the tumor is poorly limited from surrounding tissue and frequently infiltrates adjacent mucous membranes, bones and nerves. HCCC is a low-grade tumor and rarely metastases, so the prognosis of the disease is very good. We have taken into account malignant epithelial odontogenic neoplasm in the differential diagnosis. Hence, it is also composed primarily of clear cells, which usually occur in the anterior region of the mandible. Unlike HCCC, the nests or cords may show focal palisading of basal cells. In an absence of this typical feature, and a presence of hyalinization, the malignant epithelial odontogenic neoplasm was ruled out. To establish the histopathological diagnosis of HCCC, metastatic renal clear cell carcinoma needed also to be ruled out. We have also taken into account SRCLA, which may be difficult to differentiate, but clear cell tumors involving the maxillary or palatal structures require consideration of a sinonasal neoplasm with secondary involvement of the oral region. The main difference between SRCLA vs. HCCC is that SRCLA has no stromal hyalinization and no stromal vascularity. It often has larger clear cells than HCCC and has robust CA-IX immunostaining vs. focal positive in HCCC. It is also negative for EWSR1 rearrangement [1]. Despite all these similar tumors, it is possible to establish the correct diagnosis through meticulous histopathological and immunohistochemical analysis. HCCC cells are positive for AE1/AE3, p63, high-molecular-weight cytokeratin, CAM5.2, CK7, EMA (75%) markers. They are negative for S100, SMA, MSA, Calponin, GFAP and CK20 markers. Antonescu et al. identified a consistent EWSR1-ATF1 gene fusion in HCCC, and this molecular signature is not present in other clear cell mimics. The EWS (EWSR1) gene is involved in translocations in Ewing’s sarcoma, clear cell sarcoma, desmoplastic small round cell tumor and myxoid liposarcoma [8].
The modality of choice for treatment of HCCC is a complete surgical excision. Postoperative radiotherapy is recommended in patients that present with locoregional metastases. Radiotherapy alone, or in combination with radiosensitizing chemotherapy, may play a role in recurrent disease. However, the evidence is weak [9]. In comparison, metastatic renal clear cell carcinoma does not respond well to the radiation therapy, and it’s role is still being debated. For metastatic renal cell carcinoma, surgery on the metastatic lesion is advised if possible to relief the symptoms. Treatment with interleukin-2, interferon alpha and 5-fluorouracil may be useful in cases of residual disease after resection, although the partial response is less than 25% [10].
CONCLUSIONS
From our case, we can conclude that detailed histopathological and immunohistochemical evaluation is mandatory for its definitive diagnosis and differentiation from its counterparts. Our case suggests that complete local resection is mandatory and in case of close or positive resection margins, adjuvant irradiation therapy should be administered.
AUTHORS CONTRIBUTION
Each author has made substantial contributions to the conception or design of the work, or to the acquisition, analysis or interpretation of data for the work; participated in drafting the work or revising it critically for important intellectual content; approved the final version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
FUNDING
The authors received no financial support for the research, authorship and/or publication of this article.



