-
PDF
- Split View
-
Views
-
Cite
Cite
Tran Anh Tuan, Nguyen Minh Duc, A unique case of medulla oblongata epidermoid cyst, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa411, https://doi.org/10.1093/jscr/rjaa411
Close - Share Icon Share
Abstract
Epidermoid cysts (ECs) are slow-growing, benign tumors that represent <2% of all intracranial tumors. ECs can be divided into following two types: extra-axial and intra-axial. Extra-axial ECs are most often positioned in the cerebellopontine angle. Intra-axial ECs, which are also referred to as intraparenchymal ECs, are most commonly found in supratentorial structures, such as the frontal and temporal lobes, accounting for <2% of all intracranial ECs and are especially rare in children. In this report, we described a unique case of medulla oblongata EC in a child, to contribute this knowledge to the existing body of literature.
INTRODUCTION
Epidermoid cysts (ECs), which are congenital, benign and slow-developing lesions, represent <2% of all intracranial tumors [1]. ECs can be classified as either extra-axial or intra-axial. Extra-axial ECs are most commonly found on the cerebellopontine angle (CPA), followed by the supra- or para-sellar area, and along the subarachnoid spaces of the basal cisterns [2]. Intra-axial ECs, also known as intraparenchymal ECs, are extremely rare lesions, especially in pediatric patients, constituting only 1.5% of all intracranial ECs, and are always located in the cerebral hemispheres [3]. In the current literature, few reports have described pediatric infratentorial intraparenchymal ECs [3–7]; therefore, we aimed to describe an exceedingly rare case of a pediatric medulla oblongata EC.
CASE REPORT
A 3-year-old male, who suffered from a sudden, tonic–clonic seizure, followed by a short period of unconsciousness, was instantly transported to Children’s Hospital 2. The patient’s medical history was normal. No neurological deficits were detected during the clinical assessment, and laboratory tests and electroencephalography were within acceptable ranges. The clinician performed a brain magnetic resonance imaging (MRI) scan, with contrast agent. No lesions were recognized in the supratentorial structures. A clear boundary cystic mass (27 × 25 × 26 mm3) was located in the medulla oblongata, without perilesional vasogenic edema. Hydrocephalus was not observed. The signal intensity of the mass was low on the sagittal T1-weighted image (Fig. 1) and high on the axial T2-weighted image (Fig. 2). On coronal fluid-attenuated inversion recovery imaging, the mass was isointense relative to the parenchyma, but the intensity was higher than that of cerebrospinal fluid (CSF, Fig. 3). On susceptibility-weighted imaging, no indicators of hemorrhage or ossification were observed within the mass. The mass was partially hyperintense on diffusion-weighted imaging (DWI) and slightly hypointense on the apparent diffusion coefficient (ADC) map. The mean ADC values of the parenchyma, mass and CSF were 0.71, 1.3 and 1.59 × 10−3 mm2/s, respectively (Fig. 4). On T1-weighted imaging, with contrast enhancement, the thin wall of the mass was very slightly enhanced, and we observed a tiny nodule inside the mass that was strongly enhanced (Fig. 5). With a provisional diagnosis of pilocytic astrocytoma, the patient underwent surgery to completely eradicate the tumor. Eventually, the histopathological result revealed a typical EC (Fig. 6). The postoperative period was uneventful, and the patient was discharged after 2 weeks.
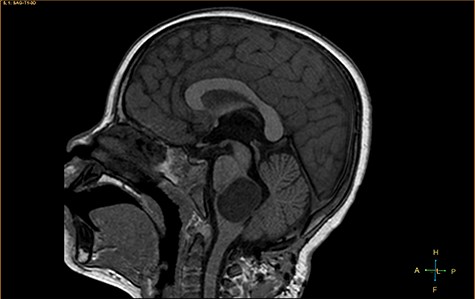
Sagittal T1-weighted image, revealing a hypointense mass positioned in the medulla oblongata.
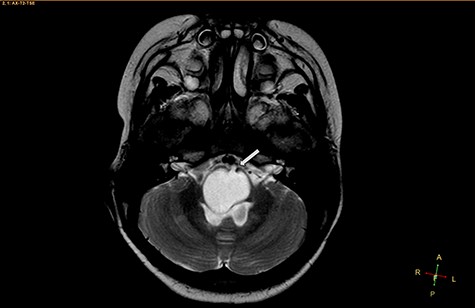
Axial T2-weighted image, showing a hyperintense, cystic mass inside the medulla oblongata. Exceptionally, the lesion appears to progress from extra-axial to intraparenchymal (arrow).
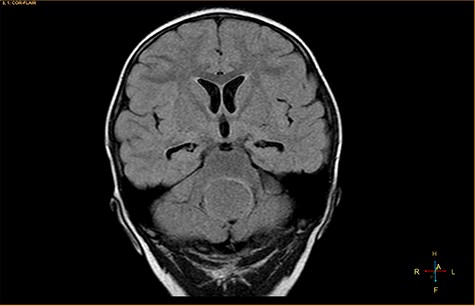
Coronal fluid-attenuated inversion recovery image, showing an isointense mass relative to the surrounding parenchyma.
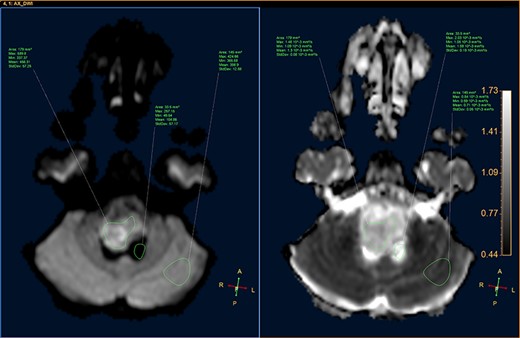
DWI image and ADC map, showing the CSF, lesion and normal-appearing parenchyma.
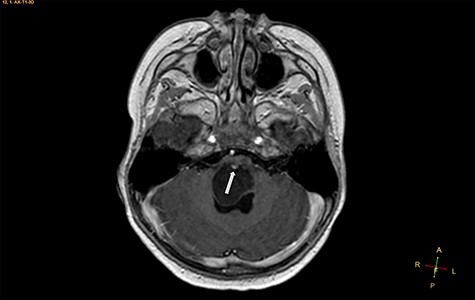
Axial T1-weighted image, with contrast agent, revealing a cystic mass, with a very small enhancing mural nodule (arrow).
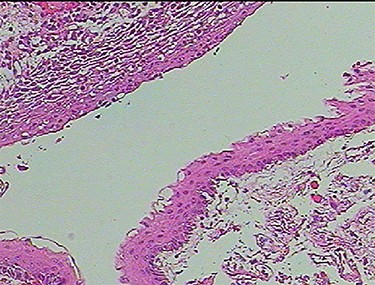
Photomicrograph of the biopsy specimen, showing that the tissue sample presents a cystic structure, packed with laminated keratin. Multilayered squamous epithelia are stratified on the cyst wall. No skin appendages are observed (hematoxylin and eosin, ×40).
DISCUSSION
Extra-axial ECs, which occur more frequently than intra-axial ECs, can develop throughout the neuroaxis and are most commonly situated in the CPA. Less common locations for extra-axial ECs include the cranial diploe, parasellar or pineal areas, middle cranial fossa and basal cisterns [1, 2, 7]. Inversely, previous studies have shown that most intraparenchymal ECs have been identified in the frontal and temporal lobes [1–3, 7]. To the extent of our knowledge, ECs located in the medulla oblongata are extremely unusual [1–7].
Two widely accepted hypotheses have been suggested to explain the establishment of extra-axial and intra-axial ECs. Theoretically, ECs form due to aberrations in ectodermal residues or the sequestration of ectodermal components during an early stage of gestational growth, between the 3rd and 5th weeks. The remnants of these ectodermal cells eventually proliferate, forming an EC [3, 4]. Retrospectively, the findings shown in Fig. 2 appear to show the progression of the lesions from the extra-axial to the intraparenchymal medulla oblongata; therefore, we have contributed additional evidence to reinforce the hypothesis regarding the primitively extra-axial root of EC development.
Histopathologically, the ECs are filled with protein, keratin and cholesterol. The cyst wall is typically covered with stratified, squamous epithelia, with the outward backing of collagen [3–8]. The appearance on MRI is heavily dependent on the chemical architecture of these intracystic components. The hypointensity observed on T1-weighted images is associated with the crystalline cholesterol pattern, whereas the hyperintensity on T2-weighted images is induced by the keratinaceous element. Occasionally, ECs appear hyperintense on T1-weighted images and hypointense on T2-weighted images, and these are referred to as white ECs [8, 9]. This specific appearance is caused by unusually high proteinaceous concentrations in the cyst. Typically, ECs do not absorb contrast agent vividly. Approximately 35% of ECs are estimated to enhance slightly and peripherally [9]. DWI is the most useful weapon that can be deployed to recognize ECs. When contrasted with the CSF, because of the superior keratinaceous and proteinaceous concentrations and the relatively little aqueous content, ECs generally appear considerably hyperintense on DWI and hypointense on ADC [9, 10].
Although some recommended imaging characteristics exist for ECs, these characteristics are not always persistent or specific [8–10]. Especially in cases of intraparenchymal ECs, provisional diagnosis can be very difficult due to similarities between EC characteristics and those of other brain neoplasms, including glioma [3–10]. In our case, we identified a medulla oblongata mass in a 3-year-old patient, with a lesion ADC of 1.3 × 10−3 mm2/s, and the identification of an enhanced mural nodule in a cystic lesion resulted in the misdiagnosis of pilocytic astrocytoma, a very common, benign, posterior fossa tumor found in children. According to Kannan et al. [10], unlike extra-axial ECs, intraparenchymal ECs do not often exhibit distinctly limited diffusivity.
Surgery is the optimal therapeutic option for ECs, and the complete removal of the tumor capsules in non-eloquent areas is advised to avoid later relapse [3–7]. In cases of intraparenchymal ECs, the cyst capsules might be thick and attached closely to adjacent tissues, which can require excision and dissection near or within eloquent structures, including the medulla oblongata, pons or midbrain; thus, the omission of EC capsule segments at vital positions may be necessary and appropriate [3–7, 10].
CONCLUSION
In summary, infratentorial, intraparenchymal ECs are exceptionally uncommon in the general population and are even rarer in the pediatric population. In the present report, an unusual presentation of a pediatric EC in the medulla oblongata, with atypical characteristics on DWI and T1-weighted imaging with contrast enhancement, resulted in the misdiagnosis of pilocytic astrocytoma. Neuroradiologists should consider that EC can appear with atypical imaging characteristics, which might imitate glioma; therefore, EC should be included in the differential diagnosis, to obtain better treatment strategies and prognosis.
AUTHORS’ CONTRIBUTIONS
T.A.T. and N.M.D. contributed equally to this work as co-first authors. T.A.T. and N.M.D. designed the project and supervised the accuracy and integrity of all stages of manuscript preparation. Both the authors were involved in the management of the patient from admission to follow-up, worked on the draft and revised it together.
STATEMENT OF ETHICS
This study was approved by the Institutional Review Board of Children’s Hospital 2 (Ref: 352/NĐ2-CĐT). Written informed consent of patient’s legal guardian for publication of this case report and any accompanying images was obtained.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



