-
PDF
- Split View
-
Views
-
Cite
Cite
Alaa Nabawi, Nader Abraham, Ayman Nabawi, Disfiguring high-flow cervicofacial arteriovenous malformations, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa435, https://doi.org/10.1093/jscr/rjaa435
Close - Share Icon Share
Abstract
Arteriovenous malformations (AVMs) are congenital vascular anomalies resulting from defects in angiogenesis. Approximately 40% of AVMs go undetected after birth and only experience the delayed clinical onset of symptoms in adulthood. AVMs are rare, representing only 1.5% of all vascular anomalies. The most common sites for the aberrant vascular nidus are the oral cavity and maxillofacial region, which represent 50% of the cases. AVMs are the most challenging and life-threatening form of vascular malformation. Exsanguination, thrombus detachment and embolization are the most hazardous operative risks. Small case series revealed a 75% recurrence rate during a 5-year follow-up, which adds another layer of complexity to their management. Large lesions in the head and neck cause deformation to the patient and present a challenge to the surgeon during their excision among vital structures and reconstruction of the 3D complex defects.
INTRODUCTION
The 2018 International Society for the Study of Vascular Anomalies (ISSVA) divided vascular anomalies into two main groups: vascular tumors and vascular malformations (Table 1).
| Vascular tumors . | Vascular malformations . | |||
|---|---|---|---|---|
| Simple . | Combined . | Of major named vessels . | Associated with other anomalies . | |
| Benign | Capillary malformations | CVM, CLM | ||
| Locally aggressive or borderline | Lymphatic malformations | LVM, CLVM | ||
| Venous malformations | ||||
| AVMs* | CAVM* | |||
| Malignant | Arteriovenous fistula* | Others | ||
| Vascular tumors . | Vascular malformations . | |||
|---|---|---|---|---|
| Simple . | Combined . | Of major named vessels . | Associated with other anomalies . | |
| Benign | Capillary malformations | CVM, CLM | ||
| Locally aggressive or borderline | Lymphatic malformations | LVM, CLVM | ||
| Venous malformations | ||||
| AVMs* | CAVM* | |||
| Malignant | Arteriovenous fistula* | Others | ||
*High-flow lesions. CVM, combined vascular malformations; CLM, capillary lymphatic malformations; LVM, lymphatic venous malformation; CLVM, capillary lymphatic venous malformation; CAVM, capillary arteriovenous malformation.
| Vascular tumors . | Vascular malformations . | |||
|---|---|---|---|---|
| Simple . | Combined . | Of major named vessels . | Associated with other anomalies . | |
| Benign | Capillary malformations | CVM, CLM | ||
| Locally aggressive or borderline | Lymphatic malformations | LVM, CLVM | ||
| Venous malformations | ||||
| AVMs* | CAVM* | |||
| Malignant | Arteriovenous fistula* | Others | ||
| Vascular tumors . | Vascular malformations . | |||
|---|---|---|---|---|
| Simple . | Combined . | Of major named vessels . | Associated with other anomalies . | |
| Benign | Capillary malformations | CVM, CLM | ||
| Locally aggressive or borderline | Lymphatic malformations | LVM, CLVM | ||
| Venous malformations | ||||
| AVMs* | CAVM* | |||
| Malignant | Arteriovenous fistula* | Others | ||
*High-flow lesions. CVM, combined vascular malformations; CLM, capillary lymphatic malformations; LVM, lymphatic venous malformation; CLVM, capillary lymphatic venous malformation; CAVM, capillary arteriovenous malformation.
Arteriovenous malformations (AVMs) are rare high-flow vascular malformations, representing 1.5% of all vascular anomalies. AVMs are congenital vascular abnormalities consisting of hypertrophied arterial inflows that shunt through a primitive vascular nidus directly into outflow veins. The arterial blood shunt away from capillary beds allows for lower vascular resistance and higher flows, leading to thicker and more tortuous inflow arteries and tortuous dilated and arterialized outflow veins. Approximately 50% of AVMs occur in the oral and maxillofacial regions, with 70% of those lesions located in the center of the face, involving the cheek, nose, ears and upper lips [2]. Unlike hemangiomas (proliferating vascular anomalies), AVMs develop via vascular ectasia rather than cellular proliferation. AVMs may increase in size proportionally with the individual asymptomatically or with only subtle findings until an accelerating event such as puberty, pregnancy or trauma promote rapid growth and subsequent development of symptoms [3].
In this series, we present our institutional experience with two different cases that highlight the heterogeneity and challenges of the aggressive and deforming nature of large AVMs in the upper cervical and maxillofacial regions during their surgical resection and subsequent reconstruction of the remaining defects.
Patient 1—sudden, unexplained cardiac arrest
A 49-year-old female patient from neighboring Libya presented with recurrent facial and neck swelling that has been growing for the last 10 years, causing her significant disfigurement, dysphagia, dyspnea and psychological distress. The patient had undergone a previous attempt of embolization, followed by surgical intervention. Both interventions ultimately failed to provide a cure and resulted in residual regrowth of the mass. The patient was admitted to our institution for a second attempt at surgical excision (Fig. 1).
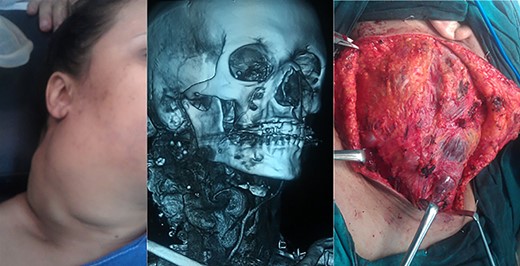
Patient #1 before surgery, magnetic resonance imaging, during surgery.
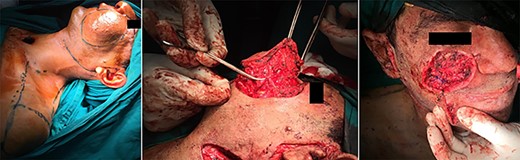
The surgery started with a cervicofacial incision, and immediately unusual excessive bleeding from the skin was noted. To our team, this excessive bleeding from the skin incision only was a familiar hallmark with this type of malformation. Control of this cutaneous and subcutaneous bleeding was challenging.
Nevertheless, deeper dissections around the mass revealed a network of extensively engorged venous plexuses with very fragile walls that bled with minor manipulations. The bleeding necessitated 2 units of blood transfusion. The incision and dissections were extended caudad to the supraclavicular region to expose the lower border of the malformation. Upon reaching the thyroid gland, we noted that the superior thyroid vessels were indiscernible from the large sinuses of the malformation approaching ~4–6 mm in diameter. Furthermore, bizarre, tortuous, large-caliber vessels were found embedded into the wall of the thyroid cartilage. After 8 hours of dissections, when the incision was extended cephalad toward the mandible, sizable vascular extensions with amorphous borders were found around the right masseter and buccinator muscles, as well as around and extensively embedded into the parotid gland. We could not discern an upper boundary for the malformation as large-caliber vessels continued to run superiorly around the nasopharynx toward the skull base.
At that team, the surgical team considered aborting the procedure. However, at that time, despite the lack of significant major vascular exsanguination, the patient suddenly and inexplicably destabilized and went into cardiac arrest, presumably due to a massive pulmonary embolism, from a dislodged thrombus. Attempts at resuscitation failed, and the time of death was declared intraoperatively. The cause of death remained presumptive as follow-up autopsy was not performed as per the family’s request.
Patient 2—successful resection and reconstruction
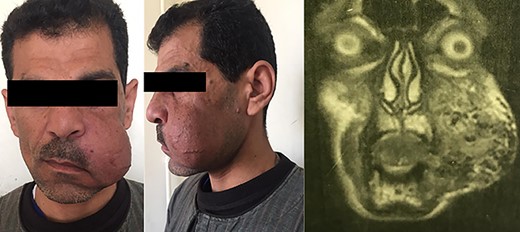
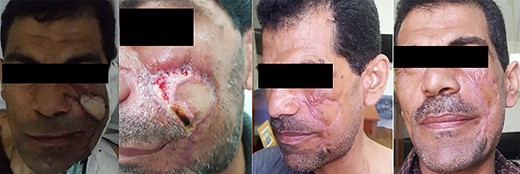
A 45-year-old male patient presented with a two-time recurrent, pulsatile, maxillofacial swelling of the left cheek. The patient’s past surgical history included two failed surgical interventions to excise the lesion 1 year and 6 months, respectively, before the current presentation. In the second recurrence, the lesion was larger in size. Other surgical histories include previous ureteric stone extraction and ureteric reimplantation. The patient’s medical and family histories were negative for similar lesions (Fig. 2).
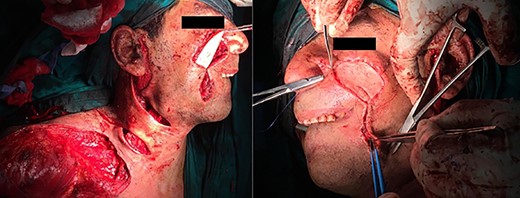
The surgery started with an extended modified Blair’s incision to allow access to ligate the left external carotid artery to minimize blood flow to the aberrant vascular nidus and decrease the risk of fatal intraoperative exsanguination (Figs 3 and 4).
Afterward, we performed a second skin incision encircling the AVM and previous surgical scars. We observed the excessive skin bleeding again with the second patient. We then dissected deeper down to the capsule of the parotid gland to allow for en-bloc excision of the tumor. Vascular extensions of the AVM were found to penetrate that plane into the superficial lobe of the parotid gland and surround the branches of the left facial nerve.
The two middle branches of the facial nerve were non-salvageable. An anchoring 3/0 Prolene suture extending from a hole drilled in the root of the zygomatic arch to the muscles of the angle of the mouth was used to maintain postoperative facial symmetry. Following the removal of the lesion, preplanned reconstruction proceeded by creating a supraclavicular island flap. The defect was covered, and the flap skin incisions were primarily closed. The patient was discharged uneventfully on the third postoperative day.
On follow-up, the flap experienced distal necrosis that healed by secondary intention. Twenty-four-month ultrasound follow-up showed absence of any aberrant flow over or around the flap (Fig. 5).
DISCUSSION
AVMs of the head and neck region are congenital anomalies where a majority (~40%) pass birth and childhood undetected [4]. AVMs continue growing via vessel ectasia with only subtle findings until precipitating events such as puberty, pregnancy or trauma accelerate their growth [4].
Patients usually present with pain, ulceration, bleeding, dysfunction or deformity. The lesions are quite disfiguring and psychologically disturbing to the patients and may be fatal with or without surgery [3]. Due to the rarity of AVMs in the head and neck region, only limited experience is available in the literature on the optimal surgical approach and management protocol [5].
High recurrence rates via residual reexpansion, aggressive growth and local destruction are all factors that contribute to their morbidity. Lethal intraoperative exsanguination or sacrifice of vital structures (such as facial nerve) is not uncommon with sizable head and neck AVMs and must be considered preoperatively, and the patient must be well informed of the risks. Other life-threatening complications include ischemic brain injury, pulmonary embolism and compressive hematoma.
Anesthesiology support with enough blood products must be present intraoperatively [5]. Physical examination typically reveals poorly defined masses, with cutaneous discoloration, a pathognomonic palpable thrill, with or without cutaneous ulceration [4].
The definitive treatment of large malformations consists of adequate resection followed by reconstruction. In our experience, a skin incision around the tumor circumference offered better and safer exposure and much less bleeding. However, it yields a substantial defect that necessitates reconstruction, preferably preplanned. Literature differs on the utility of preligating the feeding vessels and embolization. Some reports believe that preligation and preembolization help decrease the risk of intraoperative bleeding [6], whereas others believe that they reroute the growth into a surgically inoperable area [3], such as in the first patient of this report.
The mass is usually ill defined, not only during the examination but also during surgical exploration and excision. Determining the margins of the resection is most difficult, especially in the head and neck region. A conservative surgical approach usually leads to reexpansion with the recurrence of a larger mass. With an aggressive approach, the defect left is typically significant and 3D complex. In the head and neck region, reconstruction using local tissue is favored. Flap choices, whether pedicled or free, are according to the site and size of the defect. Possible options include cervicofacial advancement, deltopectoral, supraclavicular and free radial forearm flaps. For more profound defects, pedicled muscles such as the temporalis or pectoralis major covered with a split-thickness skin graft or free myocutaneous anterolateral thigh flap are rapid and easy choices. For our second patient, we opted to use a pedicled island supraclavicular flap to cover the defect.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



