-
PDF
- Split View
-
Views
-
Cite
Cite
Yi Ying Law, Rhea Patel, Rebecca Yorke, Harold R Bailey, Jeffrey L Van Eps, A case of infiltrative cecal endometriosis with appendiceal obliteration and lymph node involvement, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa396, https://doi.org/10.1093/jscr/rjaa396
Close - Share Icon Share
Abstract
Endometriosis is a clinical condition with a wide spectrum of severity, and a subset that includes intestinal involvement that may even mimic malignancy, making non-surgical diagnosis difficult. Cecal endometriosis is a rare finding among intestinal endometriosis. We report on 33-year-old woman with ileocecal endometriosis presenting as endoscopic prolapse of the ileocecal valve associated with a mass on cross-sectional imaging. The diagnosis was suggested intraoperatively by peritoneal endometrioma and obliteration of the appendix during laparoscopic right hemicolectomy. Pathological review demonstrated extensive submucosal, infiltrative endometriosis with mass effect and lymph node involvement. This case highlights the difficulty in preoperative diagnosis of intestinal endometriosis and the wide-ranging potential tissue effects in cases of infiltrative disease.
INTRODUCTION
Endometriosis is a gynecological condition with manifestations secondary to functional ectopic endometrial tissue outside the uterus. It affects up to 15% of women, typically during childbearing age [1]. The pelvis is most commonly affected and symptoms include dysmenorrhea, dyspareunia, infertility or chronic pain, which worsen during menses. Endometrial tissue deposits are commonly found on the ovaries (endometriomas), uterosacral ligament and pouch of Douglas [1]. Extrapelvic endometriosis most commonly affects the gastrointestinal tract, but other locations have been reported, including liver, lungs and pericardium [1]. The rectosigmoid area is the most common GI location (70–90% of cases), followed by the ileocecal area, appendix and other segments in descending order [2]. Symptoms of intestinal endometriosis may include abdominal pain, vomiting, diarrhea, constipation and hematochezia [1]. Cecal endometriosis can present as acute appendicitis, intussusception, volvulus, chronic abdominal/pelvic pain or bowel obstruction [3]. This myriad of potential symptoms makes diagnosis of intestinal endometriosis particularly challenging. The gold standard for diagnosis remains direct visualization with tissue sampling for histologic confirmation. For ileocecal endometriosis, distinguishing from entities such as Crohn disease or malignancy is often difficult, and surgical resection is frequently required.
CASE REPORT
A 33-year-old female presented for surgical evaluation of a possible ileocecal mass and abnormalities found on endoscopy. She presented to an outside hospital 4 months prior to surgical consultation with symptoms of abdominal pain, diarrhea and hematochezia. Computed tomography (CT) imaging demonstratedthickening of the distal descending and proximal sigmoid colon and punctate pneumatosis concerning for ischemic colitis. Endoscopy with mucosal biopsy confirmed the diagnosis of colonic ischemia but also noted marked edema of the cecum and ileocecal valve. Her condition improved with non-surgical supportive care and her hematologic coagulation profile subsequently normalized after stopping oral contraceptive use. Repeat colonoscopy 2 months after hospital discharge confirmed resolution of ischemia but persistent abnormalities of the ileocecal area with cecal fullness and a prolapsed ileocecal valve (Fig. 1), with only lymphoid hyperplasia on biopsy. Repeat CT imaging was obtained and showed a
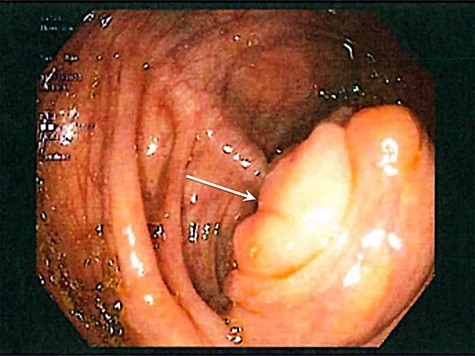
Colonoscopic image demonstrating cecal fullness with prolapsed ileocecal valve (arrow).
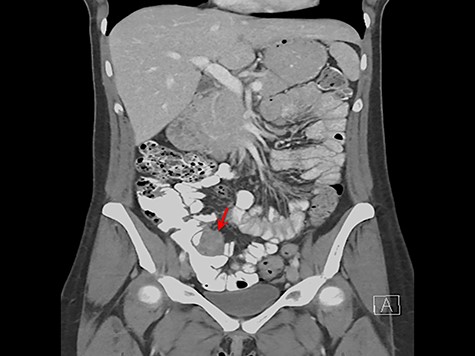
3.4 × 3.7 cm mass near the ileocecal valve (Fig. 2). Unable to rule out malignancy, surgical resection was recommended for tissue evaluation and the patient underwent laparoscopic right hemicolectomy.
Intraoperatively, a right lateral peritoneal nodule was noted upon initial diagnostic laparoscopy and excised with intraoperative frozen pathology confirming endometrioma. An uneventful right hemicolectomy was performed with a side-to-side, functional end-to-end anastomosis. Once extracorporealized, the ileocecal region was notably fibrotic and the appendix was seemingly obliterated by the infiltrative process and could not be clearly identified.
Histopathologic examination revealed a cecal endometrial mass (3.8 cm × 2.8 cm × 2.5 cm) infiltrating the muscularis propria and submucosa and ileal submucosal nodule (0.6 cm) collectively causing mass effect through smooth muscle hyperplasia and hypertrophy (Fig. 3). The appendix was confirmed to be absent and specialized immunohistochemical (IHC) staining was done to confirm that the ileocecal nodules were not involuted appendix (Fig. 4). Interestingly, several of the 45 benign lymph nodes within the surgical specimen demonstrated inclusions of endosalpingosis and endometriosis.
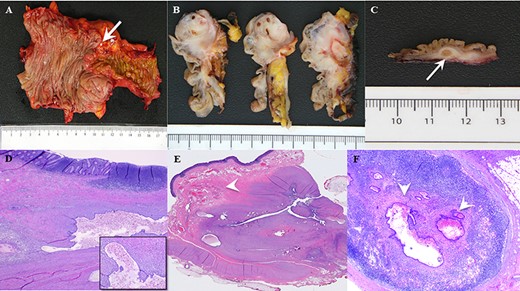
(A) Gross pathological assessment of ileocolic region demonstrates macroscopic nodularity (arrow) at the region of the ileocecal valve and appendix difficult to identify. (B) Gross pathological view after serial sagittal sectioning at the ileocecal valve demonstrates a significant infiltrative, fibrotic, mass-like effect explaining the prolapsing area witnessed on endoscopy. (C) Gross pathological section from the ileocecal region shows macroscopic evidence of a large submucosal endometrial implant (arrow) within the wall of the bowel. (D) Low-power histopathologic examination showing colonic mucosa with endometriosis in the underlying wall. Higher power microscopy (inset) shows a gland lined by benign endometrial-type epithelium with supporting endometrial stroma, containing blood and hemosiderin. (E) Histopathologic examination demonstrating cecal endometrial mass infiltrating the muscularis propria and submucosa (arrow) causing smooth muscle hyperplasia and hypertrophy, and (F) glandular endometrial tissue within a pericolonic lymph node (arrows).
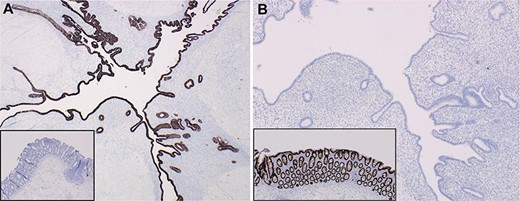
Targeted IHC staining facilitated differentiation of endometrial implants from colonic epithelium (insets) using (A) CK7+ and (B) CDX2+ staining.
DISCUSSION
There are three pervading theories describing the pathogenesis of endometriosis. The most predominant theory is‘retrograde menstruation’, whereby endometrial cells reflux and implant on ectopic sites within the pelvis and peritoneum [4]. The second is coelomic metaplasia, entailing the transformation of the peritoneum into glandular endometrium [5]. Lastly, dissemination through lymphovascular pathways is a third theory of extrapelvic endometriosis [6]. In a study reviewing 26 patients with rectosigmoid endometriosis, 42.3% had some lymph node involvement, with 36.3% lymphovascular invasion [7]. The true incidence of extragenital endometriosis with lymphatic involvement is unknown but has been reported in various locations. An increase in both the lymphatic vessel number and the expression of lymphangiogenic growth factors as seen in Table 1 has been demonstrated in various types of endometriosis, including ovarian endometriomas, peritoneal implants and infiltrative lesions [6].
Comparative expression of lymphangiogenic growth factors and their receptors in the endometrium of women with endometriosis versus unaffected women
| Levels of expression . | Growth factor/receptora . | Function . | References . |
|---|---|---|---|
| Increased | VEGF-A | Proliferation of lymphatic endothelial cells | [24, 25, 65, 82, 102] |
| VEG FR-2 | Receptor for VEGF-A, VEGF-C and VEGF-D | [82] | |
| Ang-1 | Lymphatic sprouting and hyperplasia, lymphatic vessel homeostasis | [84] | |
| Ang-2 | Postnatal lymphatic patterning and lymphatic vessel homeostasis | [84] | |
| Tie-2 | Receptor for Ang-1 and -2 | [84] | |
| HGF | Promote formation of new lymphatic vessels | [83, 85] | |
| FGF | Indirect induction of lymphangiogenesis by up-regulating VEGF-C | [86] | |
| Decreased | VEGF-C | Sprouting of lymphatic vessels, lymphatic vessel and endothelial cell development | [64, 65] |
| IGF-1 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| IGF-2 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| NRP-1 | Regulate lymphatic vessel formation and a receptor for VEGF-A | [64] | |
| Cyclical dysregulation | VEGF-D | Stimulate adult lymphangiogenesis | [64] |
| NRP-2 | Modulate developmental lymphangiogenesis | [64] |
| Levels of expression . | Growth factor/receptora . | Function . | References . |
|---|---|---|---|
| Increased | VEGF-A | Proliferation of lymphatic endothelial cells | [24, 25, 65, 82, 102] |
| VEG FR-2 | Receptor for VEGF-A, VEGF-C and VEGF-D | [82] | |
| Ang-1 | Lymphatic sprouting and hyperplasia, lymphatic vessel homeostasis | [84] | |
| Ang-2 | Postnatal lymphatic patterning and lymphatic vessel homeostasis | [84] | |
| Tie-2 | Receptor for Ang-1 and -2 | [84] | |
| HGF | Promote formation of new lymphatic vessels | [83, 85] | |
| FGF | Indirect induction of lymphangiogenesis by up-regulating VEGF-C | [86] | |
| Decreased | VEGF-C | Sprouting of lymphatic vessels, lymphatic vessel and endothelial cell development | [64, 65] |
| IGF-1 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| IGF-2 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| NRP-1 | Regulate lymphatic vessel formation and a receptor for VEGF-A | [64] | |
| Cyclical dysregulation | VEGF-D | Stimulate adult lymphangiogenesis | [64] |
| NRP-2 | Modulate developmental lymphangiogenesis | [64] |
Cited from Jerman and Hey-Cunningham [6]
aVEG F, vascular endothelial growth factor; VEG FR-2, VEGF receptor 2; Ang, angiopoietin; Tie-2, receptor tyrosine kinase; HGF, hepatocyte growth factor; FGF, fibroblast growth factor; IGF, insulin-like growth factor; NRP, neuropilin.
Comparative expression of lymphangiogenic growth factors and their receptors in the endometrium of women with endometriosis versus unaffected women
| Levels of expression . | Growth factor/receptora . | Function . | References . |
|---|---|---|---|
| Increased | VEGF-A | Proliferation of lymphatic endothelial cells | [24, 25, 65, 82, 102] |
| VEG FR-2 | Receptor for VEGF-A, VEGF-C and VEGF-D | [82] | |
| Ang-1 | Lymphatic sprouting and hyperplasia, lymphatic vessel homeostasis | [84] | |
| Ang-2 | Postnatal lymphatic patterning and lymphatic vessel homeostasis | [84] | |
| Tie-2 | Receptor for Ang-1 and -2 | [84] | |
| HGF | Promote formation of new lymphatic vessels | [83, 85] | |
| FGF | Indirect induction of lymphangiogenesis by up-regulating VEGF-C | [86] | |
| Decreased | VEGF-C | Sprouting of lymphatic vessels, lymphatic vessel and endothelial cell development | [64, 65] |
| IGF-1 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| IGF-2 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| NRP-1 | Regulate lymphatic vessel formation and a receptor for VEGF-A | [64] | |
| Cyclical dysregulation | VEGF-D | Stimulate adult lymphangiogenesis | [64] |
| NRP-2 | Modulate developmental lymphangiogenesis | [64] |
| Levels of expression . | Growth factor/receptora . | Function . | References . |
|---|---|---|---|
| Increased | VEGF-A | Proliferation of lymphatic endothelial cells | [24, 25, 65, 82, 102] |
| VEG FR-2 | Receptor for VEGF-A, VEGF-C and VEGF-D | [82] | |
| Ang-1 | Lymphatic sprouting and hyperplasia, lymphatic vessel homeostasis | [84] | |
| Ang-2 | Postnatal lymphatic patterning and lymphatic vessel homeostasis | [84] | |
| Tie-2 | Receptor for Ang-1 and -2 | [84] | |
| HGF | Promote formation of new lymphatic vessels | [83, 85] | |
| FGF | Indirect induction of lymphangiogenesis by up-regulating VEGF-C | [86] | |
| Decreased | VEGF-C | Sprouting of lymphatic vessels, lymphatic vessel and endothelial cell development | [64, 65] |
| IGF-1 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| IGF-2 | Stimulate lymphatic vessel growth by inducing VEGF-C | [81] | |
| NRP-1 | Regulate lymphatic vessel formation and a receptor for VEGF-A | [64] | |
| Cyclical dysregulation | VEGF-D | Stimulate adult lymphangiogenesis | [64] |
| NRP-2 | Modulate developmental lymphangiogenesis | [64] |
Cited from Jerman and Hey-Cunningham [6]
aVEG F, vascular endothelial growth factor; VEG FR-2, VEGF receptor 2; Ang, angiopoietin; Tie-2, receptor tyrosine kinase; HGF, hepatocyte growth factor; FGF, fibroblast growth factor; IGF, insulin-like growth factor; NRP, neuropilin.
Ultrasound and MRI can be used to diagnose intestinal endometriosis, but direct sampling by laparoscopy remains the gold standard. Mucosal involvement is rare, so colonoscopy has a limited diagnostic role. Surgical treatment depends on the depth of involvement and must balance disease eradication with preserving fertility. Shave excision and laser ablation may be used for small superficial lesions. Single infiltrating nodules <30 mm in size and less than one-third of bowel circumference can undergo discoid or full-thickness anterior wall excision, while more extensive infiltrative disease requires formal resection and anastomosis. Post-operative hormonal therapy is often effective in prolonging interval between surgeries. Progestins and gonadotropin-releasing hormone analogues may be offered to women with bowel luminal stenosis <60% who wish to avoid surgery and do not desire to conceive [8].
Our patient presented a unique diagnostic challenge, as she did not display classic symptoms of pelvic or infiltrative intestinal endometriosis. Her previous long-term use of oral contraceptives may have suppressed symptoms, while, coincidentally, her undiagnosed endometriosis may have contributed to her development of ischemic colitis at such a young age, as multiple studies have demonstrated the increased risk of hypercoagulability with endometriosis [9]. Radical resection was required for tissue diagnosis but the extent of her disease makes it likely that without surgery she would have presented with intestinal symptoms in the future.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



