-
PDF
- Split View
-
Views
-
Cite
Cite
D’Shaun D Adams, Francisco A Schwartz-Fernandes, Myoelectric prosthesis: a potential emerging therapeutic in restoring function post-arm amputation complicated by necrotizing fasciitis, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa381, https://doi.org/10.1093/jscr/rjaa381
Close - Share Icon Share
Abstract
A literature search confirmed no previous cases of an arm amputation secondary to necrotizing fasciitis (NF) being successfully treated with a myoelectric prosthesis. This report explores a case of a 55-year-old right-handed male with flexor tenosynovitis infection of the hand after a small laceration. Following infectious disease consult of the infection, a diagnosis of NF was made. Broad-spectrum antibiotics were initiated but the infection worsened after 12 hours. Two debridements with wound vacuum were undertaken in the next 48 hours. After further patient deterioration, a distal left forearm amputation was undertaken. The patient fully recovered and then underwent occupational therapy training with a myoelectric prosthesis to restore hand function. The patient was seen for follow-up 3, 6, 12 and 24 months after amputation. At 2-year follow-up, the patient was continuing rehabilitation with an occupational therapist to acclimate to the prosthesis with some gain of function in gross movement.
INTRODUCTION
Necrotizing fasciitis (NF) is a life threatening soft tissue infection characterized by necrosis of the deep layer of superficial fascia with sporadic necrosis of deep fascia and muscle. The first description of NF was posited by Hippocrates in the 5th century B.C.E [1]. NF was termed in 1952 by Wilson, characterizing the disease by calling it fascia necrosis [1].
There are current limitations to the diagnosis of NF. Current parameters for diagnosis include a combination of dissection of the affected fascia, histological and microbiological assessments [1]. There are, currently, limitations on the diagnosis of NF due to its apparent benign appearance that leads to a delay in diagnosis. Delays in diagnosis correlate to increased morbidity and mortality. Additionally, the mortality rate of NF has stagnated around 25–35% the past 20 years despite great technological, surgical and pharmacological advancement [2]. The growth of antibiotic resistance bacteria such as MRSA and VISA could potentially increase the incidence of this disease in the future.
To date, there are no previously reported cases of myoelectric prosthesis being an effective treatment for amputation complicated by NF. This report presents a case of novel treatment success after a debilitating necrotic infection. It aims to serve as a reminder of the importance of early recognition of NF along with a potential effective therapeutic post-recovery. It emphasizes that myoelectric prosthesis can be an effective tool to enhance daily living and presents the potential for a patient to regain fine motor skills that are not possible with other types of prosthesis. This specific report will focus on a case of NF in the hand and discuss the potential and barriers for myoelectric prosthesis utilization post-amputation.
CASE REPORT
A 55-year-old right-handed diabetic male presents with skin necrosis and infection on the left thumb and thenar eminence. The infection started from a 1-cm laceration obtained 6 weeks prior to his admission while working construction. At admission, the patient’s history included poorly controlled diabetes and peripheral neuropathy. The patient was started on broad spectrum antibiotics as soon as he arrived. Extensive debridement was also undertaken at admission. Cultures obtained during surgery of the initial debridement were positive for group B Streptococcus.
No improvement was observed in the next 12 hours, with expansion of the area of necrosis in the superficial and deep fascia, expanding necrosis of the left thenar eminence and medial side of the hand. The lack of improvement prompted two consecutive debridements with wound vac application in the following 48–72 hours. Between debridements, intravenous antibiotics were utilized but with poor response. As the infection worsened, the patient’s health deteriorated with increased fever, leukocytosis, septicemia and renal failure. Distal left forearm amputation was undertaken as necrosis worsened.
After amputation, the patient improved and was evetually discharged. Two years after surgery, the patient continued rehabilitation training with an occupational therapist to adjust to the myoelectric prosthesis. The patient described the training as difficult and had gain some function in moving the prosthetic arm. The patient still had goals of improving his function with the prosthesis (Figs 1 and 2).
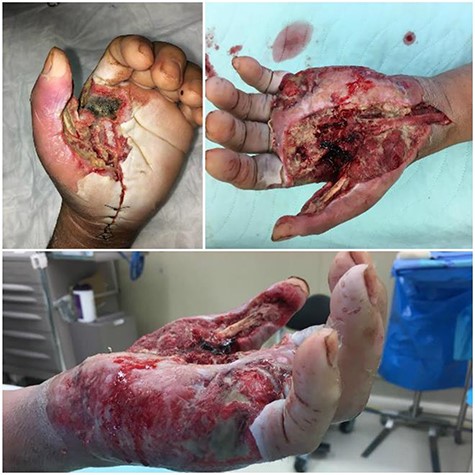
Progression of the case through subsequent procedures. Necrosis of the skin, superficial and deep fascia, expanding necrosis of the left thenar eminence and medial side of the hand. Dorsal and medial views of the hand after the second and third debridements undertaken are presented as well. A horseshoe abscess furthered necrosis throughout the hand.
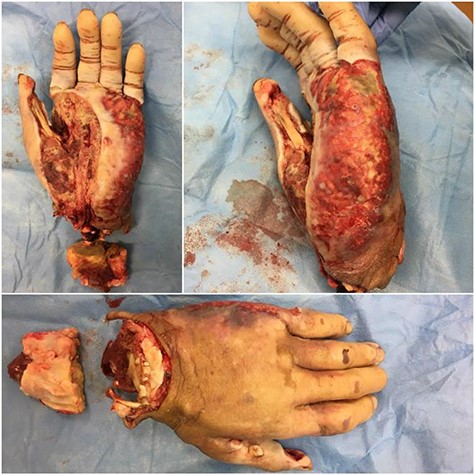
Photographs of hand immediately after the distal forearm procedure. Necrosis spread through an infected horseshoe from the thenar space to the ulnar side/hypothenar eminence of the hand. Ventral, medial and dorsal views of the hand are presented.
DISCUSSION
Treatment for NF in the hand includes surgical debridement, nutritional support and intravenous antibiotics. Due to the aggressive nature of this disease, misdiagnosis often leads to a late stage presentation of NF where broad spectrum intravenous antibiotics are no longer useful. Intervention for NF often does not occur until antimicrobials are not working, sepsis develops and ascending infections of cutaneous tissue occur [3].
Several risk factors have been identified for NF. Alcoholism, diabetes, cancer, obesity, peripheral vascular disease, liver cirrhosis, chronic renal disease, lacerations/puncture wounds, drug abuse and peptic ulcer disease are common comorbidities with NF [4, 5]. Diseases that suppress the immune system or any traumas that compromise the cutaneous tissue are also risk factors for NF [3]. The most common route of admission of NF in the hand is by contact to an open burn or cut on the hand. However, proper wound care and handwashing can prevent NF in the extremities [1, 5]. Results support that the most important factors for a positive treatment outcome are patient age and early occurrence of surgical debridement [1, 4].
Moreover, research on the prognosis of patients utilizing prosthetics post-amputation of necrotic limbs is another area of interest. This case presents a patient who regained some gross motor movement with the use of a myoelectric prosthesis. This therapeutic has the potential to be a great tool to improve the quality of life in patients that undergo amputations after a life-threatening infection. The use of myoelectric sensors measures action potentials and amplifies controls for prosthetic motors. The myoelectric prosthesis is the most functional device for an upper limb amputee and gives the patient the feeling as if they are regulating the same nerves to move before amputation and have reported to have increased dexterity, grip and force [6]. Studies also report that a myoelectric prosthesis has higher rates of satisfaction with cosmetic appearance, psychosocial and social adaptation when compared with body-powered prostheses [7]. Additionally, myoelectric prosthesis has been shown to reduce the incidence of phantom limb pain in transradial amputees [7], a phenomenon that afflicts up to 50% of upper-limb amputees [8].
Historical barriers for myoelectric prosthesis as a device have included cost, the amount of training, durability and comfort. With the improvements of 3D printing technology, the cost, durability and comfort of these prostheses will potentially improve over time with more efficient and customizable production measures [9]. Improvements in efficacy in myoelectric prosthesis can potentially improve mortality outcomes in patients with NF.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



