-
PDF
- Split View
-
Views
-
Cite
Cite
Akshay J Patel, Saifullah Mohamed, Yassir Iqbal, Ashok Kar, Gopal Soppa, A combined approach to correct posterior left ventricular aneurysm, aortic stenosis and coronary artery disease, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa356, https://doi.org/10.1093/jscr/rjaa356
Close - Share Icon Share
Abstract
Ischaemic heart disease and aortic stenosis are potentially life-threatening conditions. A post-infarct left ventricular aneurysm, when combined with the above, is particularly hazardous. We present a case where all three conditions occurred simultaneously and describe the surgical approach undertaken to attempt correction. The patient underwent aneurysmectomy together with aortic valve replacement and two-vessel coronary artery bypass grafting. The aneurysm was excised with direct linear closure of the walls using a Teflon-buttressed interrupted mattress suture technique. Post-operatively, ventricular systolic function was good (LVEF 40%) together with a well-seated aortic valve showing no paravalvular leaks. This case highlights the importance of meticulous removal of thrombus from the aneurysm and everting the edges thereby eliminating a thrombogenic surface and the risk of embolic stroke. The restorative procedure itself serves to underline the importance of ventricular shape in the effective functioning of the myocardium for sustaining an adequate stroke volume with normalized physiology.
INTRODUCTION
A left ventricular aneurysm (LVA) is defined as a distinct area of abnormal left ventricular diastolic contour with systolic dyskinesia or paradoxical bulging, such that the left ventricular ejection fraction (LVEF) is reduced [1]. True aneurysms tend to occur with bulging of the full thickness of the left ventricular wall, whereas a false aneurysm involves a left ventricular wall rupture contained by surrounding pericardium. In 1881, LVAs were recognized to be a consequence of coronary artery disease and 95% of true LVAs today are indeed a consequence of ischaemic heart disease [2]; however, other causes have been recognized such as trauma, Chagas disease, sarcoidosis as well as the congenital occurrences [1]. The incidence of LVA in post-infarct patients has varied between 10 and 35%, with a steady reduction in the absolute incidence following the advent of immediate revascularization following myocardial infarction [1].
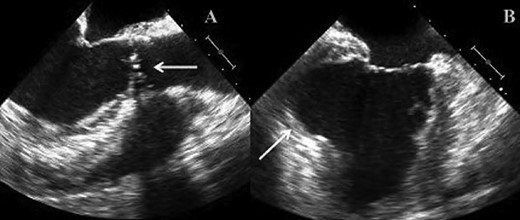
(A) TOE image showing bicuspid stenotic aortic valve. (B) TOE image demonstrating left ventricular apical aneurysm.
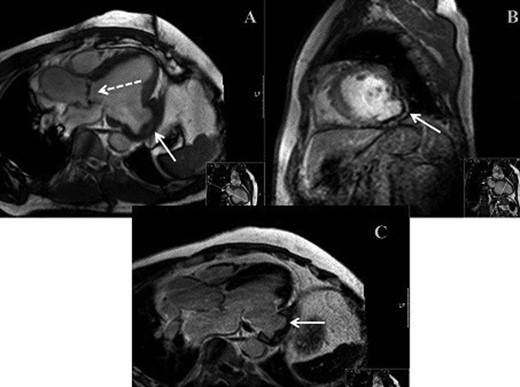
(A) MRI demonstrating bicuspid aortic valve (dashed arrow) and LVA (solid arrow). (B) MRI demonstrating parasagittal view of LVA. (C) MRI demonstrating mid-axial view of LVA.
The left ventricular shape has been defined as a prolate ellipsoid, with a unique architectural arrangement of the myocardial fibres; longitudinal, circumferential and oblique, the latter of which is essential to the effective clockwise and anticlockwise torsional movements during systole and diastole [3]. Pathological states such as ischaemic heart disease induce ventricular remodelling and this disrupts the oblique orientation, resulting in ventricular dilatation and increased sphericity [3]. These architectural changes herald the onset of physical myocardial changes and left ventricular compromise. True aneurysms are bound by scarred myocardium and are seen as a late complication of transmural infarcts that have undergone early expansion. The most consistent clinical manifestation in such patients is angina given that ∼60% of such patients have concomitant coronary artery disease [4]. Symptomatic patients warrant the need for operative intervention over medical therapy.
CASE REPORT
We report on a 57-year-old gentleman who initially presented with a 6-month history of heavy central chest pain, back and shoulder pain and shortness of breath on exertion (New York Heart Association Classification (NYHA) III, Canadian Cardiovascular Society Angina Classification (CCS) II). He suffered an inferior myocardial infarction in 2007 and has a known bicuspid aortic valve under follow-up. Risk factors for ischaemic heart disease include current smoking and a positive family history of heart disease on his maternal side. On admission, a transoesophageal ECHO (TOE) (Fig. 1) and cardiac magnetic resonance imaging (MRI) (Fig. 2) were carried out and demonstrated the bicuspid aortic valve with a mixed stenosis/regurgitation pattern (moderate–severe) (Fig. 1A). In addition, marked left ventricular dilatation and an inferior LVA extending from the base to the mid-cavity of the left ventricle measuring 4.5 cm across the orifice (Fig. 1B) were seen. The aneurysm was thin walled, with dyskinetic motion with evidence of a dense thrombus adherent to the wall. The inferior wall was noted to be akinetic and non-viable. Preoperative LVEF was 60%. The angiogram showed stenosis of the right coronary artery (Fig. 3B) in the mid-segment together with distal left anterior descending disease (Fig. 3A).
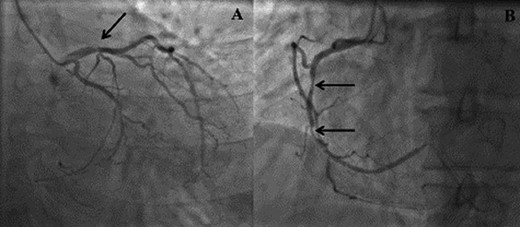
(A) Right anterior oblique (RAO) view demonstrating distal left anterior descending disease. (B) Left anterior oblique (LAO) view demonstrating mid right coronary artery disease.
Intraoperative process
The heart was elevated following application of the cross-clamp and the 5-cm posterior aneurysm opened and the thrombus within removed completely (Fig. 4). The aneurysm was excised leaving a 1-cm rim of scar tissue, which was everted, and the defect closed in a linear fashion with double-layered, Teflon-buttressed, 3.0 Prolene sutures (Johnson&Johnson® Medical Devices). The heart was placed in the normal position and the left internal mammary artery was grafted to left anterior descending artery and saphenous vein graft to circumflex artery territory (obtuse marginal 1). An oblique aortotomy was performed; the stenotic aortic valve was excised, and a bioprosthesis was implanted. Post-operatively, systolic function was good together (LVEF 40%) with a well-seated aortic valve showing no paravalvular leaks. The patient was discharged home on the ninth post-operative day with dual antiplatelet therapy but no formal Coumadin-based anticoagulation as this was felt to incur too high a bleeding risk. This was a joint decision between both clinical teams and patient.
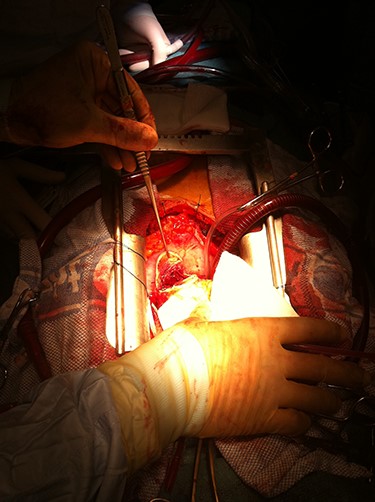
Intraoperative image demonstrating dissected LVA exposed and incised to perform intra-cavity thrombectomy.
DISCUSSION
LVAs are rare yet important differentials to consider in patients with ischaemic type cardiac chest pain. They can present as did in this patient as straightforward cardiac symptoms, the angina type chest pain, shortness of breath with exertion, pre-syncopal episodes. Furthermore, patients with a history of weakened myocardium through previous infarcts or otherwise are the category in which there will be the highest incidence of aneurysms. Moreover, given that the incidence is somewhat on the decline with the advent of the minimally invasive stenting age, one should be increasingly more vigilant for the possibilities of rarer complications. Imaging characteristics for pseudoaneurysms (false) and true aneurysms remain to be quite similar. There is a risk of ventricular arrhythmias and embolization with both types; however, the risk of rupture for pseudoaneurysms is higher than for true aneurysms. Given the fragility of the overlying pericardium in the former, this prompts the need for urgent surgical intervention for pseudoaneurysms, whereas true aneurysms are more effectively dealt with medically [5, 6].
Having said that, when presented with a severely symptomatic patient and the combined ambiguity that arises from imaging modalities when differentiating true from false aneurysms one would have to surgically intervene as a matter of urgency regardless of the aneurysm type. The procedure in this patient was albeit routine, however fundamental in principle as it aims to restore the distorted ventricular geometry back to its normal elliptical shape, and as such there is generally an increase in ejection fraction of ∼12.5%, which continues to improve over the patient’s lifetime. Owing to the nature of the case (combined presentation of aortic stenosis, LVA and coronary artery disease), the management was somewhat difficult. The LV was hypertrophied because of the increased afterload that made discerning the safely resectable aneurysm edges difficult. Ordinarily, the thin-walled, non-viable boundaries of the aneurysm are straightforward to discern but with the increased wall thickness in this setting, dissection and division of the sac was not straightforward. Moreover, the extension of the sac inferiorly resulted in a non-viable posterior territory, hence the decision not to the graft the right coronary artery, which resulted in incomplete revascularization. Intraoperatively, the heart was not manipulated until the cross-clamp had been applied to avoid the increased risk of thromboembolism from the aneurysm. Owing to the position of the aneurysm, the heart had to be lifted and as such retrograde cardioplegia was avoided to prevent damage to the coronary sinus when the heart was lifted to the antegrade position. The procedure itself aims to remodel the ventricle back to optimal orientation, and there are many techniques used to achieve this; Dor procedure (endoventricular circular patch plasty), Cooley’s linear suturing technique, surgical anterior ventricular endocardial restoration (SAVER method) and left ventricular aneurysmectomy reconstruction [5]. All procedures are different routes to achieving left ventricular geometric restoration [5].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- aortic valve
- aortic valve stenosis
- myocardium
- left ventricular ejection fraction
- myocardial ischemia
- coronary artery bypass surgery
- coronary arteriosclerosis
- left ventricular aneurysm
- aneurysm
- embolic stroke
- aneurysmectomy
- aortic valve replacement
- heart ventricle
- infarction
- polytetrafluoroethylene
- stroke volume
- surgical procedures, operative
- suture techniques
- systole
- thrombectomy
- physiology
- sitting position



