-
PDF
- Split View
-
Views
-
Cite
Cite
Schauki Mahmoud, Hosam Salman, Massive bleeding of a jejunal gastrointestinal stromal tumour: a rare case of a life-threatening presentation, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa355, https://doi.org/10.1093/jscr/rjaa355
Close - Share Icon Share
Abstract
A jejunal gastrointestinal stromal tumour (GIST) is a rare neoplasm of the gastrointestinal tract. Massive bleeding due to a jejunal GIST is a diagnostic challenge and could present as a life-threatening situation needing urgent intervention. A 54-year-old woman presented with episodes of melaena and haematochezia for the previous 3 days. An oesophagoduodenoscopy was inconclusive. A contrast computed tomography (CT) scan showed a well-defined extraluminal mass in the proximal jejunum suggestive of a bleeding GIST. Her haemodynamic state deteriorated despite initial supportive therapy including a blood transfusion. An urgent laparotomy to excise the jejunal mass was performed. Histopathology and immunohistochemical studies confirmed the diagnosis. She subsequently received adjuvant imatinib therapy. She has remained symptom free at 5 months post-op. We therefore present a rare case of obscure massive gastrointestinal bleeding due to a jejunal GIST. The CT scan was the most effective investigation to detect the source of bleeding in this case.
INTRODUCTION
Gastrointestinal stromal tumours (GISTs) are rare neoplasms arising from the interstitial cells of Cajal in the gastrointestinal (GI) tract. GISTs account for only 0.1—3% of all neoplasms in the GI tract [1]. The majority of GISTs are located in the stomach (60–70%), whereas 20–30% are found in the small intestine and rarely in the oesophagus, colon, rectum and retroperitoneum [2].
Intermittent GI bleeding is the most common manifestation of a gastrointestinal stromal tumour (GIST) (42%), but massive life-threatening bleeding needing urgent intervention is a rare presentation [3]. Abdominal pain (20%) and obstruction (10%) are other potential manifestations. In all, ~20% of GISTs are asymptomatic and are discovered incidentally during abdominal imaging or intraoperatively [2].
Immunohistochemical staining for CD 117 antigen is positive in ~95% of GISTs and is considered the mainstay of diagnosis [3].
CASE REPORT
A 54-year-old woman presented with a history of multiple episodes of melaena and haematochezia accompanied by colicky abdominal pain and fatigue for the previous 3 days. Other than hypertension she did not have any other comorbidity. On primary physical examination she was pale and had a slight tachycardia but was normotensive. Abdominal examination was unremarkable. Digital rectal examination confirmed melaena stool mixed with fresh blood. Laboratory tests revealed a low haemoglobin of 7.2 g/dl. Clotting tests were normal. The patient was commenced on IV fluids and a transfusion with packed red blood cells. Emergency gastroscopy to the third part of the duodenum did not show any evidence of active bleeding. She continued to pass large amounts of melaena and fresh blood and her haemoglobin was noted to have dropped to 5.4 g/dl. A colonoscopy was decided against. She received further packed red blood cells and underwent an urgent computed tomography (CT) scan with oral and IV contrast. This showed a well-defined homogenous extraluminal mass (4 × 3.5 cm) in the proximal jejunum with mesenteric neovascularisation. There was no evidence of intra-abdominal metastases or enlarged lymph nodes (Figs 1 and 2). These findings were most suggestive of a bleeding GIST. Angiography and embolisation facilities were not available in our institution. The patient was becoming unstable and so an urgent laparotomy was required.
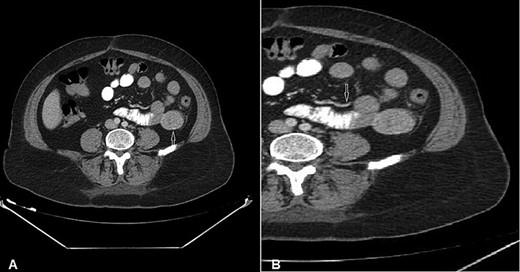
CT of the abdomen with Intravenous and oral contrast (axial section) shows: (A) 4 × 3.5 cm well-defined homogeneous extraluminal mass of proximal jejunum (white arrow) without intraperitonial metastasis or lymphadenomegaly; (B) mesenteric neovascularisation (white arrow).
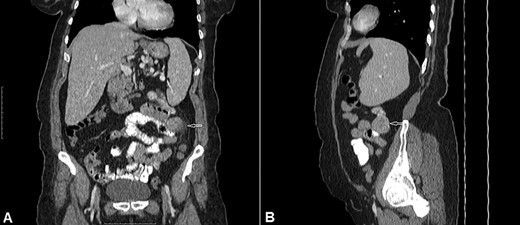
CT of the abdomen with Intravenous and oral contrast (A) coronal section, (B) sagittal section shows: well-defined extraluminal mass of proximal jejunum (white arrow).
At laparotomy we found a hypervascular 4 × 3.5 cm mass in the proximal jejunum ~15 cm from the ligament of Treitz. The mass was protruding from the anti-mesenteric border and was of a very firm consistency. The small and large bowel was seen to be loaded with blood (Fig. 3). As per the CT scan there was no sign of any intraperitoneal metastases or enlarged lymph nodes. The mass was resected with a margin of 4 cm on either side and a primary anastomosis performed. The postoperative course was uneventful and she was discharged on Day 7 post op.
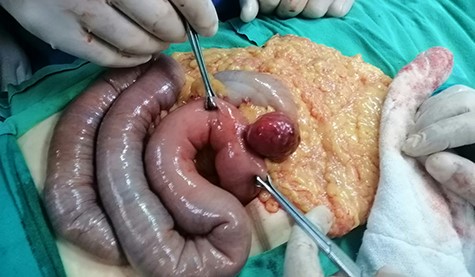
Intraoperative view: hypervascular 4 × 3.5 cm extraluminal mass of proximal jejunum. Small and large bowel are loaded with blood.
Histology showed a mixed cell type jejunal GIST with a mitotic index of <5/50 HPF—High Power Fields, clear resection margins and an intact tumour capsule (Figs 4 and 5).

Gross pathology: well-circumscribed extraluminal mass (4 × 3.5 × 3) cm; cut surface is smooth, tan and firm with lobulated appearance, hemorrhagic and necrotic areas.
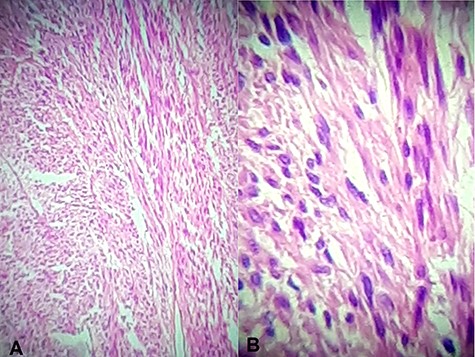
Hematoxylin and eosin stain (A) Jejunal mass at ×4 magnification, (B) jejunal mass at ×40 magnification: consist of spindle cells of varying cellularity, hyperchromatic and nuclear pleomorphism with areas of epithelioid cells (mixed type GIST).
Immunohistochemical staining for CD 117 was positive confirming the diagnosis of a GIST (Fig. 6). Molecular assessment for c-KIT D816V and PDGFRA D842V mutations (markers of resistance to adjuvant imatinib therapy) were performed and were negative. Thus adjuvant imatinib 400 mg/day was commenced.
The patient remains symptom free at 5 months follow-up and will undergo surveillance abdominal CT scans at intervals of 6 months.
DISCUSSION
Acute GI bleeding is a common presentation in medical practice and can present as a life-threatening abdominal emergency. Bleeding from the small intestinal is only found in 5–10% of patients presenting with a GI bleed [4]. The main aetiologies of small bowel intestinal bleeding include:
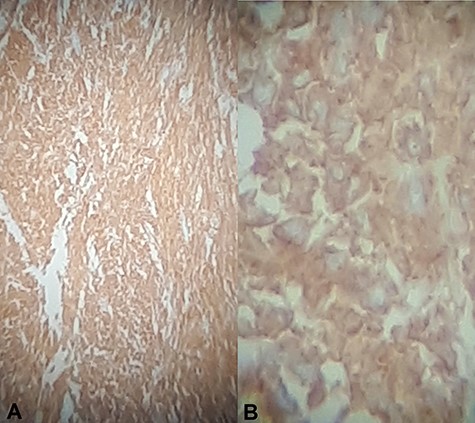
Immunohistochemical staining for CD117 (A) Jejunal mass at ×4 magnification, (B) jejunal mass at ×40 magnification is diffusely positive.
Meckel’s diverticulum and inflammatory bowel disease are common in patients <40 years old, whereas angioectasia and ulcers secondary to anti-inflammatory medications are more common in patients >40 years old [4]. There are only a few case reports of a jejunal GIST being the cause of small intestinal bleeding.
Upper and lower GI endoscopy remained the first line investigation of GI bleeding. Small intestinal bleeding is a diagnostic challenge as this region is inaccessible by conventional endoscopy. The imaging modalities required after negative upper and lower GI endoscopy include capsule endoscopy, CT angiography, conventional CT scan with IV +/−oral contrast, double balloon enteroscopy and magnetic resonance enterography [4]. CT scan features of small intestinal GISTs include a well-circumscribed extraluminal mass with homogeneous enhancement in small tumours and heterogeneous enhancement due to a necrotic centre in large tumours. Lymph node enlargement is not a feature of GIST. The liver and peritoneum are the specific sites for metastases if present [5]. In our case the intraoperative findings were compatible with the CT scan description.
As our patient became unstable, interventional angiography, even if the facilities had been available in our Institute, would not have been appropriate and so we proceeded to an emergency laparotomy.
To achieve curative treatment of a GIST, complete tumour excision with negative margins is required. Lymph node dissection is not required [2, 6]. In spite of complete excision with clear margins, GISTs tend to recur in ~50% of cases [6]. According to the Armed Forces Institutes of Pathology criteria and modified NIH criteria the tumour in our patient was classified as low risk for recurrence with no need for adjuvant imatinib therapy [6]. However recent research demonstrates that GI bleeding is due to tumour perforation through the mucosa and therefore should be treated similarly to cases where there has been tumour rupture, which changes the risk to high. These studies suggest that GI bleeding could be an independent risk factor for recurrence and therefore these patients require adjuvant treatment with imatinib [2, 7, 8].
Most GISTs (70–80%) have KIT mutations and less commonly have mutations in platelet-derived growth factor receptor alpha (PDGFRA) (10%) and wild-type (10–15%) [6]. GISTs with c-KIT D816V and PDGFRA D842V mutations are highly resistant to imatinib therapy [6, 9, 10]. These were negative in our patient and thus adjuvant imatinib therapy was initiated.
CONCLUSION
Small intestinal GISTs should be considered when managing patients with obscure GI bleeding. Contrast CT scan seems to be an effective tool to diagnose small intestinal bleeding GISTs that are inaccessible by conventional endoscopy.
ACKNOWLEDGMENT
We would like to thank Mr. Michael Ibrahim for editing English language of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- hemodynamics
- computed tomography
- hemorrhage
- gastrointestinal bleeding
- hematochezia
- immunologic adjuvants
- pharmaceutical adjuvants
- blood transfusion
- laparotomy
- melena
- diagnosis
- jejunum
- neoplasms
- gastrointestinal tract
- imatinib mesylate
- psychotherapy, supportive
- gastrointestinal stromal tumor
- massive hemorrhage
- supportive care
- histopathology tests



