-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Meyer, Andreas Johann Molnar Koszka, Phillipe Abreu, Raphaella Ferreira, Marcelo Callado Fantauzzi, Vanderlei Segatelli, Andre Ibrahim David, Chronic pancreatitis with ductal stones in the pancreatic head treated by surgery: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa352, https://doi.org/10.1093/jscr/rjaa352
Close - Share Icon Share
Abstract
Pancreatic duct stones are direct sequelae of chronic pancreatitis (CP) and can occur in ∼50% of patients. Selection of the appropriate treatment method for pancreatic duct stones depends on location, size and number of stones. We present a patient with upper abdominal pain and weight loss for the previous 3 months. Diagnostic workup detected a chronic inflammation of the pancreas with stone in the main pancreatic duct and a nodular lesion in the head of the pancreas. Endoscopic retrograde cholangiopancreatography was performed without success. Given the rise in incidence and prevalence of CP, the potential complications and high mortality rate, it is imperative that physicians understand the risk factors, disease process and management of this disease. Pancreaticoduodenectomy in patients with CP is a feasible option for the treatment of focal cystic lesions to the head of the pancreas associated to pancreatic stone in selected cases.
INTRODUCTION
Chronic pancreatitis (CP) involves progressive inflammatory and fibrotic changes of the exocrine pancreas owing to repeated episodes of acute inflammation over a long period [1]. Main risk factors for CP include alcohol abuse, smoking, gene mutations, autoimmune syndromes, metabolic disturbances, environmental conditions and anatomical abnormalities, which in turn may lead to formation of pancreatic duct stones in ∼50% of patients [2].
CP has been found to be associated with ∼50% mortality rate within 20–25 years of diagnosis due to factors including infection, malnutrition and complications from recurrent pancreatitis [3]. Additionally, CP is a risk factor for pancreatic cancer and increases the risk at least 13.3-fold [4].
Imaging can play a key role in diagnosing CP. Imaging modalities including transabdominal ultrasound, endoscopic ultrasound (EUS), computerized tomography scan and magnetic resonance imaging (MRI) can be used to detect morphological changes in the pancreas. However, diagnosing CP from imaging alone is challenging, given that morphologic changes may not appear on imaging until later in the disease. The most recent guidelines recommended reserving the use of EUS for patients in whom diagnosis is inconclusive [4].
If medical therapy fails, more invasive measures of pain management can be utilized. Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy, extracorporeal shock wave lithotripsy and placement of stents can be performed in patients found to have obstructive stones or ductal stenosis [5]. Selection of the appropriate treatment method for pancreatic duct stones depends on the location, size and number of stones [6].
Surgical options exist when medical and minimally invasive therapies fail. These procedures include decompression, drainage or resection. Surgical decompression is reserved for patients with refractory pain with a dilated pancreatic duct. Resection is indicated in patients found to have pancreatic cancer or inflammatory mass causing postobstructive CP, and in patients with small duct disease where a decompression procedure would not be helpful [7].
CASE REPORT
A 57-year-old man presented with upper abdominal pain and weight loss for the previous 3 months. He had no comorbidities and denied history of diabetes, alcoholism or previous episodes of acute pancreatitis. Physical examination revealed no abnormalities. Blood tests and tumor biomarkers results were normal.
MRI, 1 week after, showed a benign well-circumscribed nodular lesion in the head of the pancreas without contrast enhancement and associated pancreatic stones (Fig. 1).
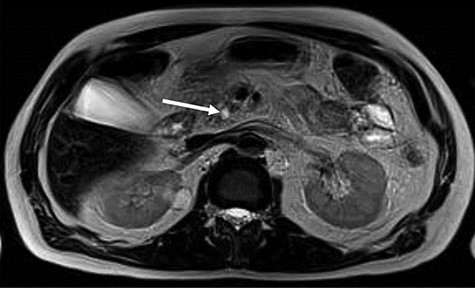
MRI—hyposignal focus within the main pancreatic duct in the cephalic region, measuring 0.6 cm, associated with irregular dilation of the upstream duct, suggesting stone (arrow).
EUS, 15 days after, suggested chronic inflammation of the pancreas with multiple stones (≤9.0 mm) in the main pancreatic duct leading to ascending dilatation and tortuosity of the main pancreatic duct. Moreover, it presents a solid formation in the head/uncinate transition on its dorsal face (Fig. 2). A biopsy was performed and the cytologic result was inconclusive.
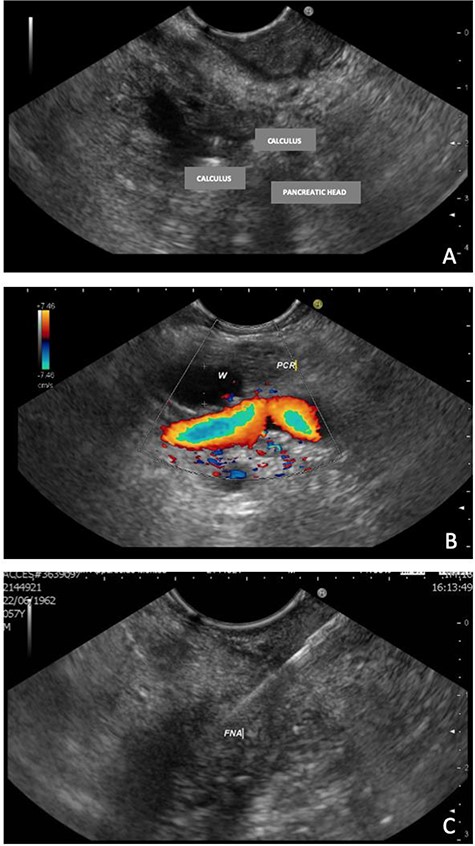
EUS—solid formation in the head/uncinate transition on its dorsal face. (A) EUS demonstrating multiple calculi and the pancreatic head; (B) EUS with evidence of a 7-mm Wirsung duct (PCR = pancreas; W=Wirsung duct). (C) Fine needle aspiration (FNA) of the nodular lesion.
Two months after, patient persisted with refractory intermittent abdominal pain crisis. Then, ERCP was performed intended to extract stones and drain the main pancreatic duct. Unfortunately, the procedure was unsuccessful for stones removal.
Pancreatoscopy was not an option available. Considering the ductal obstruction related to the nodular lesion, pancreatic decompressive drainage procedure was not considered the best option for the definitive long-term treatment of the patient.
A pancreaticoduodenectomy was then performed (Fig. 3). A drain was placed in the surgical area.
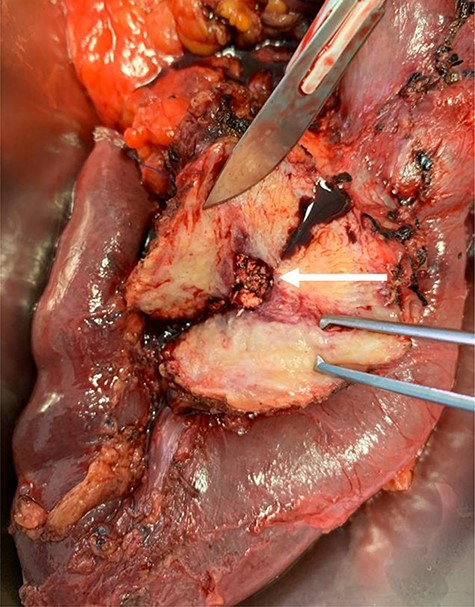
Surgical specimen demonstrating pancreatic ductal stones (arrow)
The patient had an uneventful postoperative course and was discharged in the postoperative Day 10. Final pathology report demonstrated CP with chronic inflammatory infiltrate (Fig. 4).
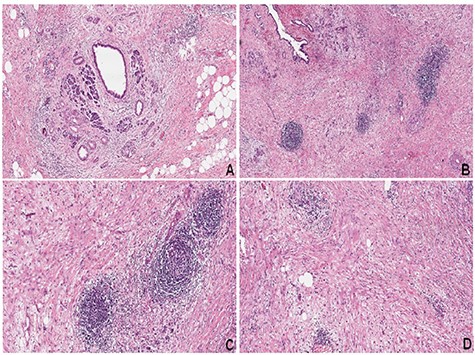
(A) CP with acinar atrophy, fibrosis and chronic inflammatory infiltrate (hematoxylin–eosin, HE). (B–D) Extensive areas of fibrosis with deposition of dense collagen. Presence of chronic inflammatory infiltrate with a predominance of lymphocytes and reactive lymphoid follicles (HE)
At 6 months of follow-up, the patient was completely asymptomatic.
DISCUSSION
Most patients with CP have abdominal pain, with a reported prevalence of 50–90% [2, 4]. When complications such as pancreatic duct stones occur, elevated pancreatic ductal pressure exacerbates pain, and it often leads to the use of analgesic drugs, loss of weight and reduced quality of life, and it can induce other complications, such as diabetes, steatorrhea and jaundice [7]. In addition, those with pancreatic duct stones are at ∼27-fold higher risk of developing pancreatic cancer than healthy individuals [8].
Pancreatic duct stones are direct sequelae of CP and can occur in ∼50% of patients. Their prevalence increases over time to reach 50 and 100%, at 5 and 14 years after the onset of the disease, approximately [2, 4]. Selection of the appropriate treatment method for pancreatic duct stones depends on the location, size and number of stones [9].
The common clinical scenarios that warrant endoscopic intervention in patients with CP are intraductal stones in the region of the pancreatic head, main pancreatic duct stricture and symptomatic pseudocyst. Large stones usually need extracorporeal shockwave lithotripsy (ESWL). Studies of ESWL plus ERCP to clear the pancreatic duct stone fragments have not shown any added benefit compared with ESWL alone [10].
However, some studies show that the surgical approach can be superior to symptoms control in some cases. A pancreaticoduodenectomy or even a total pancreatectomy may be considered when CP is located in the pancreatic head. In a recent Japanese study, it was demonstrated that the most commonly used technique consists of pancreaticoduodenectomy without pylorus preservation (10.8%), pancreaticoduodenectomy with pylorus preservation (7.8%), Frey’s surgery (52.7%), Beger’s surgery (1.2%), Puestow’s surgery (3%), Partington’s surgery (14.4%) and distal pancreatectomies (10.1%) [7].
In this case, we opted not to perform a total pancreatectomy due to the absence of lesions to the body and tail of the pancreas, rather a very focal cystic lesion to the head, causing fluid stasis and stone formation inside the main pancreatic duct. Although potentially a morbid procedure, a pancreaticoduodenectomy can provide long-term symptomatic control with good quality of life for highly selected patients.
Given the rise in incidence and prevalence of CP, the potential complications and high mortality rate, it is imperative that physicians understand the risk factors, disease process and management of this disease. Importantly, a better understanding of the mechanism behind CP is necessary in order to develop therapeutic options to prevent the progression of CP and the development of diabetes mellitus and pancreatic cancer.
Pancreaticoduodenectomy in patients with CP is a feasible option for the treatment of focal cystic lesions to the head of the pancreas associated to pancreatic stone in selected cases.
CONFLICT OF INTEREST STATEMENT
None declared.



