-
PDF
- Split View
-
Views
-
Cite
Cite
Sebahat Kaya, Stefanie Zimmer, Peer Wolfgang Kämmerer, Palatal steatocystoma simplex—a rare oral finding at an even rarer location, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa347, https://doi.org/10.1093/jscr/rjaa347
Close - Share Icon Share
ABSTRACT
Steatocystoma is a rare, benign cyst that mostly originates from a dermal sebaceous gland. It can be divided into steatocystoma multiplex—with multiple locations—and steatocystoma simplex occurring at a single site. The lesion is mostly located on the skin but can be found on other locations as well. This is the first case report of steatocystoma simplex that was found in the palate of a 37-year-old male. After resection with small safety margins and local wound dressing, no recurrence was detected during a follow-up of 1.5 years.
INTRODUCTION
Steatocystoma is a rare, benign sebaceous gland cyst that results from a mutation in the keratin-17 (K17) gene. As a result of this gene mutation, the keratin intermediary filament network is disrupted [1]. Steatocystoma multiplex shows multiple asymptomatic cysts which appear as bright nodules in the armpits, on the groin, on the trunk and on the extremities. In contrast, the even rarer isolated form of this disease is described as steatocystoma simplex [2]. An intraoral steatocystoma simplex is a very rare tumor. We report the first case of steatocystoma simplex which affected the soft palate.
CASE REPORT
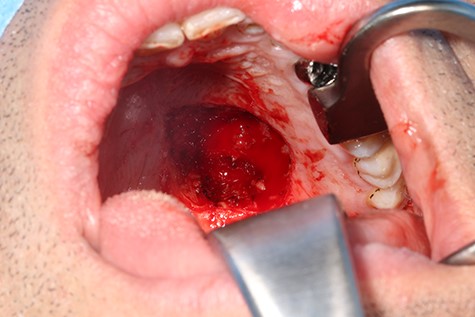
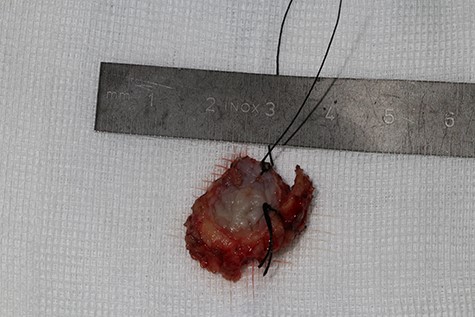
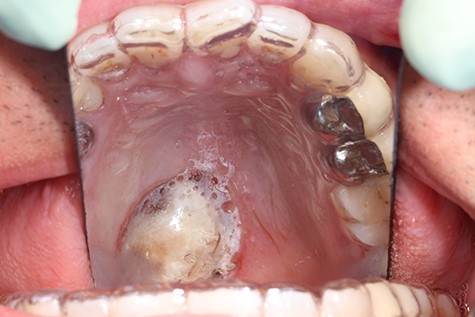
A 37-year-old patient with a recurrent, histologically confirmed steatocystoma of the palate was referred to our clinic. The patient’s medical history had already included two local removals of the tumor. Clinically, in the transition from hard to the soft palate, an asymptomatic, about 1 cm large, round, circumscribed and soft lesion was seen (Fig. 1). Magnetic resonance imaging was performed for further diagnosis and for assessment of spread and tissue infiltration of the finding. Here, a submucosal, 8 × 10 × 8 mm large tumor of delimited growth could be verified. It was T1 hypertense, T2 inhomogeneous partly hypointense, partly isointense with a significant signal drop in the T2 fat saturation (Fig. 2). There was no evidence of bony infiltration. The lesion was resected with a safety margin of 1–2 mm protecting the nerve and the greater palatine artery up to the palatal bone (Figs 3 and 4). As a secondary granulation of the defect was intended, the wound was first treated with a cellulose tamponade and tranexamic acid gel to prevent bleeding. An acrylic splint was then incorporated as a pressure bandage and wound protection (Fig. 5). The histopathological examination of the removed specimen showed a soft and glandular tissue covered with squamous epithelium with manifestations of a cystic, regressively changed epithelial lesion, which was compatible with the clinically known steatocystoma (Fig. 6). Follow-up examinations at 3-month intervals showed a good wound healing with complete restitution without evidence of recurrence after 1.5 years.
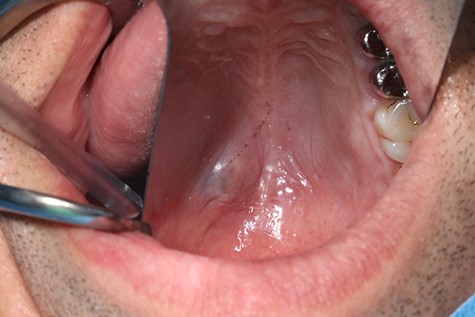
Clinical appearance of the tumor in the transition from hard to the soft palate on the left side of the mouth
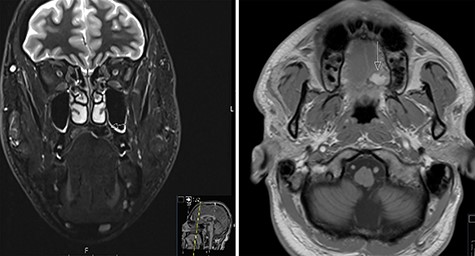
MRI scans of steatocystoma simplex involving the left side of the palate
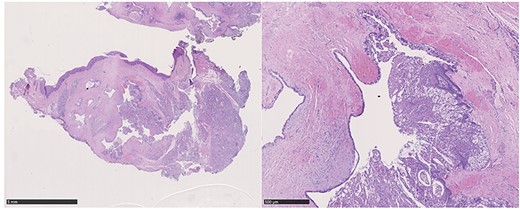
Hematoxylin–eosin staining: glandular tissue covered with squamous epithelium with manifestations of a cystic, regressively changed epithelial lesion
DISCUSSION
Steatocystoma multiplex was first described by Jamieson in 1873 as a disease of the sebaceous glands which is characterized by numerous dermal cysts scattered over the body [3]. Although most cases are sporadic, a mutation in the K17 gene was found to be responsible, leading to disruption of the keratin intermediate filament network [4]. Steatocystoma multiplex is also described as a characteristic feature of pachyonychia congenita type II, a rare autosomal dominant ectodermal dysplasia that also results from a mutation in the K17 gene [5]. Most cases of steatocystoma occur in the second and third decade of life. However, congenital cases as well as cases in the seventh decade of life were observed as well. Clinically, steatocystoma multiplex shows multiple asymptomatic cysts which appear as bright nodules especially in the armpits, on the groin, on the trunk and on the extremities [6]. In contrast, the rarer, isolated form of this disease is described as steatocystoma simplex [2]; however, the two forms do not differ histopathologically. Little is known about the intraoral manifestation of this disease since only one case of steatocystoma simplex in the oral cavity has been published so far. Just as seen in the presented case, an asymptomatic, round, clearly circumscribed and soft lesion was described that was located in the maxilla between the canine and the first premolar [7].
Primarily, the diagnosis of steatocystoma multiplex consists of medical history and the clinical examination. In case of suspected malignancy, a pathological examination of the tissue, for example via punch biopsy, is recommended [8]. In the case of intraoral findings, diagnostic imaging should be carried out in addition to the medical history, clinical examination and histopathological examination. In case of palatal location, possible benign and malignant tumors of the minor salivary glands—which account for <25% of intraoral salivary gland neoplasms—should be considered as well.
Treatment options include the carbon dioxide laser, modified surgical technique and cryotherapy [9]. Intraoral localization of such findings, as described in this case, can limit the patient’s quality of life. The therapy of choice should focus on the complete surgical removal of the cyst with the entire epithelium for histopathological examination. In addition, a reduced occurrence of recurrences can be assumed [10].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



