-
PDF
- Split View
-
Views
-
Cite
Cite
Mang Yik Foo, Baldwin P M Yeung, Jeremy T H Tan, Laparoscopic resectional oesophago-gastroplasty: a novel technique for minimally invasive treatment of large high gastric lesser curve GIST involving gastroesophageal junction, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa346, https://doi.org/10.1093/jscr/rjaa346
Close - Share Icon Share
Abstract
A paramount factor in selecting the operative approach for gastric gastrointestinal stromal tumours (GIST) is tumour location. Tumours located high along the lesser curve of the stomach pose a challenge in laparoscopic resection. A 56-year-old lady presented with per rectal bleeding and loss of weight. Endoscopic and radiological investigations revealed a large gastric GIST located over the lesser curve with proximal margin <1 cm from the gastroesophageal junction (GEJ). We present the steps of a novel technique for laparoscopic resectional oesophago-gastroplasty to resect large high gastric lesser curve GIST involving the GEJ.
INTRODUCTION
The role for laparoscopic resection of gastric gastrointestinal stromal tumours (GIST) has continued to expand. Laparoscopic resections not only have been proven to be technically feasible while achieving R0 (microscopically margin-negative) excision but also maintaining oncologic principles [1]. A paramount factor in selecting the operative approach for gastric GIST is tumour location [2]. However, to date, there are no consensus guidelines on selection of surgical approach pertaining to location of tumour [2]. Due to their anatomical location, tumours along the lesser curve of the stomach and peri-gastroesophageal junction (GEJ) pose a challenge in laparoscopic resection. The lesser curve has limited length; this region of the stomach lacks redundancy [1], and resection close to the GEJ poses risks of trauma to the lower oesophageal sphincter [3]. Large peri-GEJ tumours that are not amenable to limited resection requires consideration of either proximal gastrectomy with jejunal interposition or total gastrectomy [4], which carry significant morbidity [3]. This report outlines a novel technique pertaining to this issue, developed to resect large high gastric lesser curve GIST involving the GEJ.
CASE REPORT
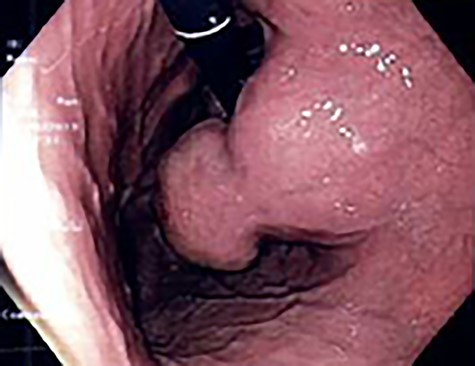
A 56-year-old lady presented with per rectal bleeding and 4 kg weight lost over 1 month. Oesophagogastroduodenoscopy (OGD) performed revealed a large gastric submucosal tumour over the lesser curve (Fig. 1). The proximal margin of the tumour is <1 cm from the GEJ, whereas the distal margin is in the mid lesser curve, about 5 cm proximal to the incisura. Endoscopic ultrasound with fine-needle aspiration performed confirmed the submucosal tumour to be GIST.
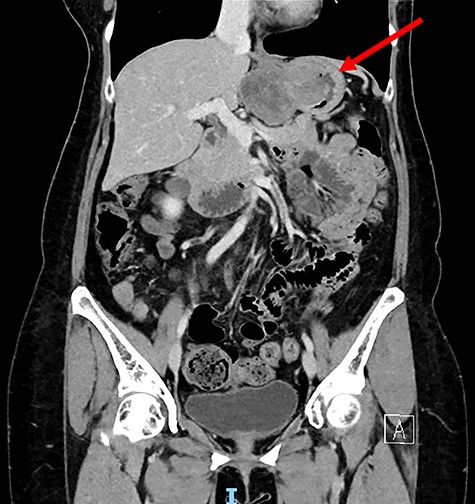
Computed tomography thorax abdomen pelvis performed showed a 4.2 × 8.0 × 5.3 cm transmural mass with prominent luminal and exophytic components at the gastric cardia and GEJ (Fig. 2). There were otherwise no enlarged lymph nodes or distant metastases.
Laparoscopic resectional oesophago-gastroplasty (LROG)
The patient is placed under general anaesthesia and then positioned in the French (supine split-leg) position. The surgeon stands in between the patient’s legs, the first assistant or camera assistant stands on the patient’s right whereas the second assistant stands on the left (Fig. 3).
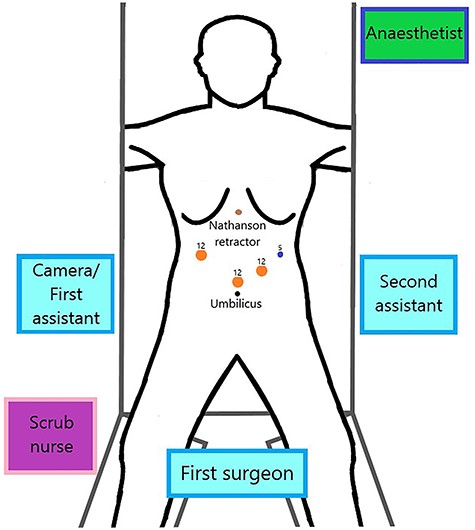
The abdominal cavity is accessed via direct optical entry technique, above and to the left of the umbilicus. A 12 mm Xcel trocar is inserted, which is used as a camera port and insufflation of the pneumoperitoneum. Under direct visualisation, two trocars (one 12 mm and one 5 mm) are inserted in the left upper quadrant and a 12 mm trocar is inserted in the right upper quadrant (Fig. 3). The Nathanson liver retractor is inserted subxiphoid.
Intraoperatively, the tumour is located along the lesser curve in the posterior wall (Fig. 4a). For tumour exposure, the lesser omentum and oesophageal hiatus were fully dissected with harmonic scalpel. Circumferential full-thickness resection around the GIST with 1 cm cuff of normal stomach tissue is performed to achieve R0 resection. As the tumour involves just below the GEJ, three quarters of the distal oesophagus was opened circumferentially in order to completely remove the tumour (Fig. 4c). The postresection defect is reconstructed by primary closure method. The gastric edge is opposed to the oesophagus with 3-0 absorbable V-lock sutures (Fig. 4d). Anterior fundoplication is then performed with two interrupted 0 ethibond sutures (Fig. 4e).
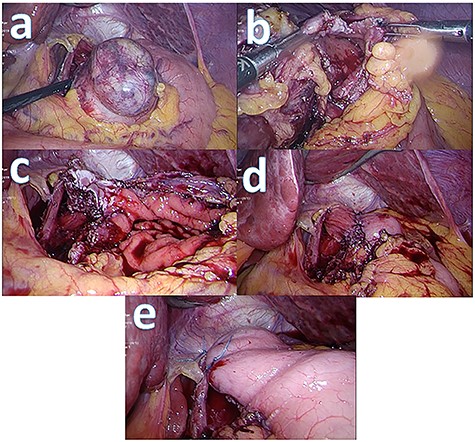
Intraoperative picture series: (a) tumour prior to resection; (b) partially resected tumour with gastrotomy made; (c) post tumour resection with oesophagotomy and gastrotomy made; (d) post oesophago-gastroplasty; (e) anterior fundoplication.
OGD is performed to confirm anastomotic integrity. A nasogastric tube is inserted, after which a Blake drain is inserted with its distal end positioned at the oesophageal hiatus. The specimen is retrieved via extraction bag through the periumbilical port site.
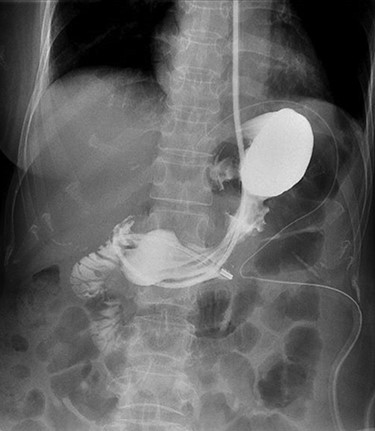
Operative time was 200 min. Postoperatively, she was kept on sips of water until postoperative day (POD) 5 which she had water-soluble contrast study that showed no anastomotic leak (Fig. 5). Feeds and diet were escalated accordingly, and she was discharged on POD 7. She was reviewed in outpatient clinics 3 weeks upon discharge and had no dysphagia or reflux symptoms. The histopathology report was GIST, of high mitotic rate and margins were clear.
DISCUSSION
GISTs are the commonest mesenchymal tumours of the gastrointestinal tract [5]. Regardless of tumour size (including intramural lesions ≤2 cm), every GIST is considered to have malignant potential [6]. The ESMO Clinical Practice Guidelines define the standard of care for localised resectable GIST as complete surgical resection, without dissection of clinically negative lymph nodes [7–8].
For gastric GISTs, an essential factor in deciding the operative approach is tumour location [2]. The favourable tumour locations include fundus, greater curve and anterior wall of the stomach [9] as laparoscopic wedge resections can be performed. Unfavourable tumour locations are lesser curve, GEJ, antrum, pylorus and posterior wall of the stomach [9]. One significant cause for conversion of laparoscopic to open surgery is tumours located at GEJ or lesser curve [2, 9]. Tumours located at the GEJ are technically challenging to resect, especially laparoscopically, and often require proximal or total gastrectomy in order to avoid gastric inlet stricture, functional compromise and gastric denervation [3]. More than often, despite a small tumour, a major gastrectomy with significant morbidity is required to remove it [3].
The most prominent benefit of LROG compared to the conventional surgeries is that it allows for minimally invasive function-preserving limited resection. The stomach’s function as a reservoir and its digestive and endocrine roles are preserved. It also prevents postgastrectomy syndrome which significantly impacts quality of life.
The postresection defect reconstruction step of LROG requires a considerable level of laparoscopic expertise. This step is crucial in ensuring the patency and diameter of the oesophageal outlet is preserved during reconstruction. The anterior fundoplication performed subsequently serves as a prophylactic anti-reflux procedure to restore the competency of the lower oesophageal sphincter as it could have possibly been destructed during tumour resection.
CONCLUSION
In the hands of an experienced laparoscopic surgeon with the ability to handle technically demanding aspects of the surgery, LROG is a safe and feasible technique to resect GISTs located at the high lesser curve of the stomach and peri-GEJ. This technique benefits patients not only as it is minimally invasive, but also permits function-preserving limited resection without compromising oncologic principles. With appropriate patient and tumour selection, patients will experience advantageous outcomes.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.



