-
PDF
- Split View
-
Views
-
Cite
Cite
Yazan Al Salhi, Andrea Fuschi, Gennaro Velotti, Lorenzo Capone, Sara Aversa, Cosimo de Nunzio, Natale Porta, Vincenzo Petrozza, Antonio Carbone, Antonio Luigi Pastore, Robot-assisted retroperitoneal lymphadenectomy in patient with type I papillary renal cancer recurrence after 5 years of follow-up, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa336, https://doi.org/10.1093/jscr/rjaa336
Close - Share Icon Share
Abstract
Papillary renal cell carcinoma (PRCC) is a rare cancer and is the second most frequent histologic type among all renal cell carcinoma, accounting for up to 15%. A 72-year-old man underwent a right radical nephrectomy 7 years ago with final histopathology diagnosis of type 1 PRCC with negative surgical margins. Five years after surgery, computed tomography scan imaging showed the presence of multiple masses suspicious for node recurrences disease localized in the renal lodge, in the inter-aorto-caval space, at the iliac vessels bifurcation and right common iliac vessels. Patient underwent a robotic retroperitoneal lymphadenectomy. The histopathological examination confirmed the recurrence of type I papillary renal cancer in all the specimens. No further recurrences have been observed at 24-month follow-up after surgery. This report is the first describing a robot-assisted minimally invasive surgical excision for type I papillary renal cancer nodal and renal fossa recurrences.
INTRODUCTION
Papillary renal cell carcinoma (PRCC) is the second most frequent histology type among all renal cell carcinoma, accounting for up to 15% [1]. PRCC is classified into two groups, type 1 and type 2, depending on nuclear features and growth pattern characteristics [2].
Patients with type 1 PRCC are diagnosed at a lower stage and present lower nuclear grade when compared with type 2 [3]. Type 1 reported a higher cancer-specific survival and a reduced association with inferior vena cava (IVC) thrombus compared with type 2 [4].
We present a case of a papillary type 1 renal cell carcinoma lymph nodes recurrence after 5 years of follow-up. To the best of our knowledge, this is the first report in literature describing a papillary type 1 recurrence after such a long follow-up time.
CASE PRESENTATION
A 72-year-old man underwent a right radical nephrectomy 7 years ago for a 10 cm mesorenal mass with a final histopathology diagnosis of type 1 PRCC, stage pT2a with negative surgical margins.
Five years after the surgery, computed tomography (CT) scan imaging showed the presence of multiple masses suspicious for node recurrences disease localized in the renal lodge, in the inter-aorto-caval space, at the iliac vessels bifurcation and right common iliac vessels. Positron emission tomography (PET)-CT scan confirmed the abnormal uptake in the same lesions with a maximal standardized uptake value of 3.2.
Patient underwent robot-assisted retroperitoneal lymphadenectomy. Patient was positioned on the left flank side. A three-arm robotic approach (12 mm camera port located 4 cm lateral to the umbilicus; 8 mm port in the epigastrium for robotic arm #1; 8 mm port in the right lateral mid-abdomen for robotic arm #2; and a 12 mm and 5 mm port inferior and, respectively, lateral and medial to the camera port for the assistant) with a 30-degree down lens (da Vinci Surgical System Si, Intuitive Surgical, Sunnyvale, CA) was used. Robotic monopolar scissors and Maryland bipolar were primarily used with a robotic prograsp for retraction.
The procedure started with the excision of the right common iliac lymph nodes and then moving to the iliac bifurcation lymph nodes. The surgery continued with the access to the interaortocaval space, with the excision of a 4 cm mass where the IVC was carefully defined with meticulous dissection to prevent inadvertent violation (Fig. 1). At last, the procedure continued with the excision of retroperitoneal fat of the right renal fossa and of a 2 cm peritoneal nodal recurrence.
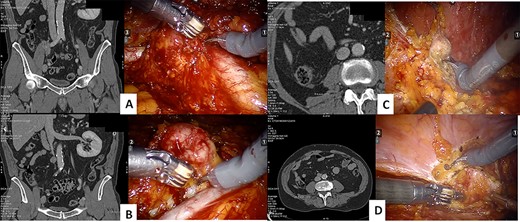
CT scan and intraoperative image of the: iliac bifurcation lymph node recurrence (A), the interaortocaval space lymph node recurrence (B), the renal fossa recurrence (C) and the peritoneal recurrence (D)
The operative time was 114 min, and estimated blood loss was 200 ml. The abdominal drainage was removed at first postoperative day. The length of hospital stay was 3 days. No complications occurred.
At hematoxylin and eosin staining, fibrous connective tissue with multiple foci of a proliferation consisting of numerous papillary projections with fibrovascular axis replete with frothy macrophages and delimited by a single layer of small cellular elements, with ovoid nucleus and inconspicuous nucleolus and weakly basophilic cytoplasm (Fig. 2).
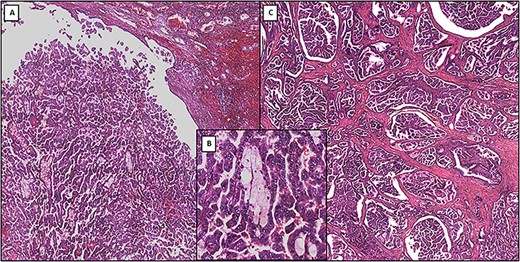
(A) Low-power photomicrograph showing primary papillary renal cell carcinoma (original magnification ×4). (B) Medium-power photomicrograph showing the detailed structure of primary papillary renal cell carcinoma: papillae formed by delicate fibrovascular cores that contain foamy macrophages (original magnification ×20). (C) Low-power photomicrograph showing metastatic papillary renal cell carcinoma (original magnification ×4)
The removed lesions confirmed on histopathological examination the recurrence of type I papillary renal cancer in all the nodal packages.
Three months after surgery, patient was treated with systemic immunotherapy (sunitinib), which was stopped 1 month later because of severe adverse events (general weakness associated to nausea and vomiting). Patient is still under follow-up and had no recurrences were observed 24 months after surgery.
DISCUSSION AND CONCLUSION
PRCC reports a better prognosis after surgical treatment when compared with clear cell renal cell carcinoma, and most cases are linked to a lower stage and describe a lower risk of cancer-specific and overall mortality [1, 5, 6].
PRCC is characterized by a significant heterogeneity observed in both histology level and clinical evolution, with the postulation of two morphologically different entities linked to cytogenetic diversity [2, 3]. The two histology subtypes lead to different rates of recurrence and survival, where stage and lymph node involvement represent the most important predictors of relapse, as reported by several studies [7–10].
Margulis et al. [7] studied 245 patients surgically treated for PRCC describing both tumor stage and lymph node involvement as independent prognostic factors of disease-specific mortality.
Compared with clear cell renal tumors, PRCC is characterized by predisposition for regional lymph node spread with synchronous metastases at diagnosis [8], and these findings are more frequently observed in tumors >8 cm [9].
Ledezma et al. [10] investigated 627 PRCC patients undergoing surgery and reported that 48 subjects experienced relapse. Ten out of 27 patients with type 2 PRCC exhibited metastatic lymph nodes, whereas most type 1 PRCC patients (11 of 21) reported pulmonary metastases.
Open surgical management has been most extensively documented, but the feasibility of laparoscopic management with or without hand assistance has been described only on a limited basis. We present the first description of minimally invasive surgical excision for type I PRCC nodal recurrence, as well as renal fossa recurrence.
Our present study shows that patients treated with surgical excision for isolated retroperitonial lymph nodes (RPLN) recurrences may experience durable progression free survival and cancer free survival. The patient at his last follow-up (24 months) was studied with PET-CT scan that confirmed the absence of local and distant recurrences. The impact of adjuvant therapy for type 1 PRCC (such as tyrosine kinase inhibitors) is still under investigation in literature, and only few reports are on subtype 1 PRCC not allowing to conclude about the role of systemic therapy in this tumor. Furthermore, in our case, the therapy was discontinued after only 30 days for the onset of adverse events.
Present data show that surgical resection of isolated RPLN recurrence from PRCC may afford select patients durable cancer control.
ETHICS APPROVAL
All procedures in this study were approved by the Ethical Committee of the Sapienza University of Rome, Department of Medico-Surgical Sciences and Biotechnologies.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- surgical procedures, minimally invasive
- computed tomography
- cancer
- renal cell carcinoma
- follow-up
- ilium
- lymph node excision
- retroperitoneal space
- surgical procedures, operative
- diagnosis
- diagnostic imaging
- kidney
- lymph node dissection
- radical nephrectomy
- surgical margins
- excision
- papillary renal cell carcinoma
- histopathology tests
- renal cancer



