-
PDF
- Split View
-
Views
-
Cite
Cite
Shuhei Kawabata, Hajime Nakamura, Takeo Nishida, Masatoshi Takagaki, Nobuyuki Izutsu, Tomofumi Takenaka, Eisaku Terada, Satoru Oshino, Haruhiko Kishima, Transnasal flow reduction in endovascular treatment for anterior cranial fossa dural arteriovenous fistula, Journal of Surgical Case Reports, Volume 2020, Issue 10, October 2020, rjaa327, https://doi.org/10.1093/jscr/rjaa327
Close - Share Icon Share
ABSTRACT
Transarterial embolization (TAE) is a useful option for anterior cranial fossa–dural arteriovenous fistula (ACF–dAVF) as endovascular devices have progressed. Liquid agents are usually injected via a microcatheter positioned just proximal to the shunt pouch beyond the ophthalmic artery; however, high blood flow from the internal maxillary artery (IMA) often impedes penetration of embolic materials into the shunt pouch. Therefore, reducing blood flow from the IMA before embolization can increase the success rate. In the present case, to reduce blood flow from branches of the IMA, we inserted surgical gauze infiltrated with xylocaine and epinephrine into bilateral nasal cavities. Using this method, we achieved curative TAE with minimal damage to the nasal mucosa. Transnasal flow reduction is an easy, effective and minimally invasive method. This method should be considered in the endovascular treatment of ACF–dAVF, especially in patients with high blood flow from the IMA.
INTRODUCTION
For anterior cranial fossa–dural arteriovenous fistula (ACF–dAVF), transarterial embolization (TAE) is a useful option as endovascular devices have progressed [1–5]. Liquid embolic agents are usually injected from a microcatheter positioned just proximal to the shunt pouch. However, TAE can be performed only in selected patients with suitable angiographic anatomy, to reduce complications such as vision loss [1].
ACF–dAVFs have two main feeding arteries such as the ophthalmic artery (OphA) and the distal internal maxillary artery (IMA) [5]. In TAE via the OphA, penetration of the embolic material into the shunt pouch can be impeded by high blood flow from the distal IMA.
We achieved curative TAE by temporarily decreasing the blood flow from the IMA branches by inserting gauze infiltrated with xylocaine and epinephrine into the nasal cavities. We named this easy and minimally invasive method as ‘transnasal flow reduction’ (TFR).
CASE REPORT
A patient in their 70s with extracranial lymphoma was incidentally found to have ACF–dAVF via head computed tomography and magnetic resonance angiography. Digital subtraction angiography (DSA) confirmed ACF–dAVF with multiple feeding branches, arising from bilateral OphAs, distal IMAs and the left middle meningeal artery (MMA), with cortical venous reflex (Borden type III, Cognard type IV) (Fig. 1). At the patient’s request, we chose endovascular, rather than surgical, treatment. We injected a 20% N-butyl-2-cyanoacrylate (NBCA)–lipiodol mixture into the fistula through bilateral ethmoidal arteries and the left MMA after we placed coils at the terminal branch of the right OphA. However, we could not achieve full penetration into the fistulous connections because of pressure secondary to high flow from the IMA branches, which resulted in incomplete obliteration (Fig. 1). Four months later, we repeated TAE by temporarily reducing nasal blood flow by inserting gauze infiltrated with xylocaine and epinephrine into the nasal cavities. After introducing the guiding catheter, an endonasal surgeon inserted X-ray-detectable surgical gauze infiltrated with 1% xylocaine and epinephrine (1:10 000) into bilateral nasal cavities using a nasal speculum, while paying full attention to avoid damage to the nasal mucosa. Then, we confirmed that the gauzes were placed in appropriate locations in the upper nasal cavity under fluoroscopic guidance. Immediately after insertion, we were able to confirm decreased blood flow from the IMA using DSA (Fig. 2). After this procedure, we navigated a DeFrictor Nano Catheter (Medico’s Hirata, Osaka, Japan) into the terminal branch of the OphA, which was connected to the dorsal nasal artery. Even though there was still a distance from the tip of the microcatheter to the shunt pouch, the NBCA reached the shunt point and penetrated the venous portion (Fig. 3). Follow-up DSA demonstrated complete obliteration of the ACF–dAVF, and blood flow in the nasal mucosa from the IMA branches recovered normally (Fig. 4).
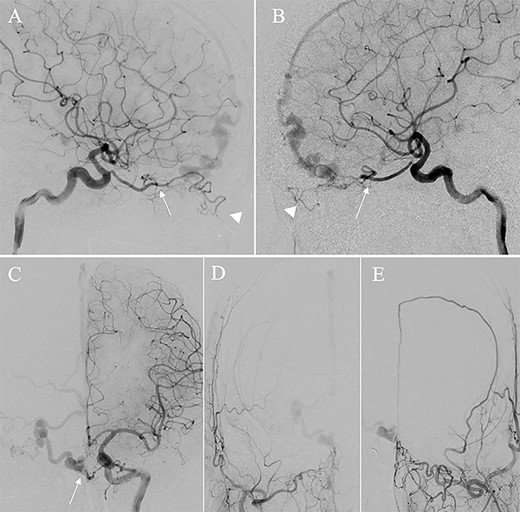
Right (A) and left (B and C) internal carotid artery angiography before initial treatment showing ACF–dAVF with feeding arteries arising from branches of the OphAs; ethmoid artery (arrow) and dorsal nasal artery (arrowhead) (D and E). Bilateral external carotid artery angiography before initial treatment, anteroposterior view, showing the ACF–dAVF fed by bilateral distal internal maxillary arteries and the left middle meningeal artery.
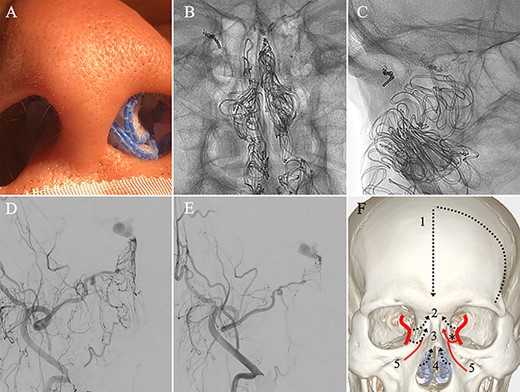
(A) Inserting gauze infiltrated with epinephrine into bilateral nasal cavities. (B and C) Radiograph showing the gauze inserted toward the upper nasal cavity. (D) DSA before inserting the gauze, anteroposterior view, showing blood flow from the right distal IMA. (E) DSA immediately after inserting the surgical gauze, anteroposterior view, showing decreased blood flow from the IMA. (F) Schematic drawing of remaining (red solid line) and occluded feeding arteries (black dotted line) after inserting the surgical gauze. Schema describing that the left dorsal nasal artery (asterisk) remained patent after embolizing the left middle meningeal artery (1), bilateral ethmoid arteries (2) and the right dorsal nasal artery (3), and blood flow from bilateral distal IMAs (4) decreased using our transnasal flow reduction method. (5) Angular artery.
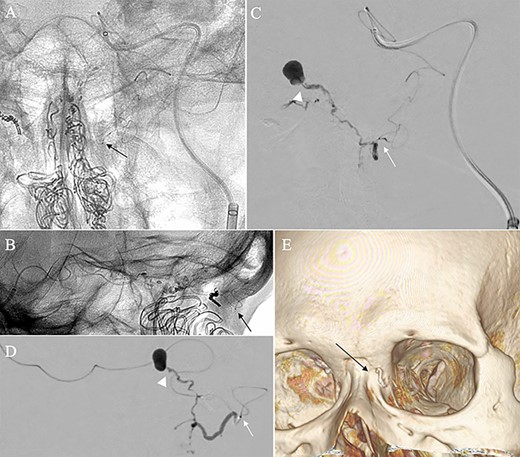
Angiographic images, anteroposterior (A) and lateral (B) views, showing the microcatheter (arrow) navigated into the terminal branch of the left terminal branch of the ophthalmic artery, which connects to the dorsal nasal artery. Digital subtraction angiography, anteroposterior (C) and lateral (D) views, showing NBCA injected via the microcatheter (arrow) penetrating into the shunt point (arrowhead) without reflux of the NBCA. (E) Three-dimensional reconstruction of the skull showing the NBCA cast (arrow), which was embolized via the dorsal nasal artery.
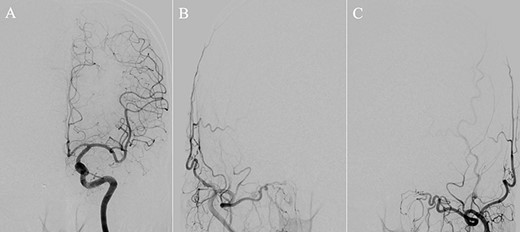
(A) DSA, anteroposterior view, showing complete obliteration of the anterior cranial fossa–dural arteriovenous fistula. (B and C) DSA, anteroposterior view, showing restored blood flow from the internal maxillary artery branches.
DISCUSSION
TAE for ACF–dAVF is a useful option following recent developments in endovascular devices; for example, flexible and trackable microcatheters, microguidewires, embolic agents and imaging devices [1–5]. TAE can be performed safely and successfully only in patients with less tortuous dilated OphAs; therefore, only 19–46% of patients with ACF–dAVF undergo TAE, and the success rate is 0–63% [1, 3, 5].
When injecting embolic materials from branches of the OphA, reflux of liquid agents into the central retinal artery or internal carotid artery should be avoided. Although there is no consensus on the optimal embolic material for TAE for ACF–dAVF [1, 3], Onyx (ev3, Irvine, CA, USA) was reported to be associated with a higher risk of complications [1]. Therefore, we decided to use NBCA in our patient.
ACF–dAVF has two main feeding arteries such as the OphA, with bilateral supply in 93% of patients, and the distal IMA, in 62–66% of patients [5]. If we expect curative embolization via the distal branch of the OphA, the blood supply from IMA branches is an important factor. In patients with high blood flow from IMA branches through the nasal mucosa, the related pressure might repulse the embolic agent and impede effective injection into the shunt pouch. Therefore, high blood flow in the nasal mucosa must be decreased before TAE.
Regarding methods used to reduce blood flow, TAE for the IMA branches using liquid agents or coils is not ideal. Liquid agents may damage the nasal mucosa, and coils may fail to decrease blood flow because of the rich vascular network in the nasal mucosa [6, 7]. Therefore, we devised TFR, which worked well, as we expected, without damaging the nasal mucosa. In the endonasal procedure, damage to the nasal mucosa should be avoided because this may induce marked bleeding because of the high flow from the IMA and secondary to heparinization. Furthermore, an endoscope may be required in patients with deviated nasal septa.
In this report, we described a novel and effective technique to achieve curative TAE for ACF–dAVF with minimal damage to the nasal mucosa. To our knowledge, this is the first report confirming the usefulness of TFR for TAE in patients with ACF–dAVF. This method should be considered in the endovascular treatment of ACF–dAVF, especially in patients with high blood flow from the IMA.
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHORS’ CONTRIBUTIONS
S.K. and H.N. drafted the article. All authors critically revised the article.
REFERENCES
- lidocaine
- epinephrine
- surgical procedures, minimally invasive
- vascular flow
- embolization
- maxillary artery
- mucous membrane of nose
- ophthalmic artery
- surgical procedures, operative
- nasal cavity
- embolism
- dural arteriovenous fistula
- shunt
- anterior fossa of cranial cavity
- endovascular procedures
- fluid flow



