-
PDF
- Split View
-
Views
-
Cite
Cite
Catarina Moura, Maria Inês Leite, Rafaela Parreira, Armando Medeiros, Primary breast lymphoma, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz405, https://doi.org/10.1093/jscr/rjz405
Close - Share Icon Share
Abstract
Primary breast lymphoma is a rare entity that accounts for only 1% of malignant breast neoplasms. It is characterized by the presence of a breast lump, with or without associated regional adenopathy and without systemic involvement. Imaging findings are nonspecific, and diffuse large B-cell lymphoma is the most common histological type. Given its rarity, there is still no consensus on the best approach, with chemotherapy being the most accepted treatment. This article aims to present a literature review as well as to present a clinical case.
INTRODUCTION
Breast lymphoma is a rare entity representing about 0.04–1% of malignant breast neoplasms, less than 1% of non-Hodgkin’s lymphomas and about 1.7% of all extra-nodal lymphomas. In turn, it may occur as extra-nodal primary disease, primary breast lymphoma or as a result of secondary systemic dissemination [1].
Primary breast lymphoma was first defined by Wiseman and Liao in 1972. The following diagnostic criteria were postulated: the breast as the site of presentation, breast tissue in close relation with lymphomatous infiltration, absence of disseminated disease beyond the ipsilateral axillary lymph nodes and no previous lymphoma diagnosis [2].
This definition remains unchanged until today. However, it only comprises stage I (breast-limited) and stage II (limited to breast and ipsilateral axillary ganglia) tumors [3].
Its pathophysiology is still unknown, but it is thought that it may derive from mucosa-associated lymphoid tissue (MALT), lymphoid tissue adjacent to breast ducts and lobes or even from intra-mammary lymph nodes [2].
Regarding the histological type, diffuse large B-cell lymphoma is the predominant variant, although up to 44% of patients may have MALT lymphomas [1, 4]. Other less common variants are: Burkitt’s lymphoma, marginal zone lymphoma, small-cell lymphocytic lymphoma and large-cell anaplastic lymphoma [5].
The average age of diagnosis varies between 60 and 65 years and occurs almost exclusively in females, with few cases of primary breast lymphoma reported in men [6]. Bilateral involvement is described in about 11% of cases [5].
The clinical presentation and the imaging characteristics are no different from breast carcinoma. The presence of a palpable lump is the most common manifestation (61% of cases), usually painless and often located in the supero-external quadrant. Other signs include: pain (12%), local inflammatory signs (11%) and palpable adenopathies (25%). In about 12% of cases, it appears as an incidental finding on mammogram as the patient is asymptomatic [5].
Imaging findings are nonspecific. At mammography, most lesions correspond to hyperdense (91%) and oval (71%) masses, whereas in ultrasound, they appear as single (75%), circumscribed (50%), microlobulated (38%) and oval (50%) lesions. Generally, they are hypoechoic (87%); calcifications or spiculated margins are not frequent [7].
The diagnostic approach follows the same principles as any other breast lump with histological confirmation of the nodule and eventual suspicious adenopathies [5].
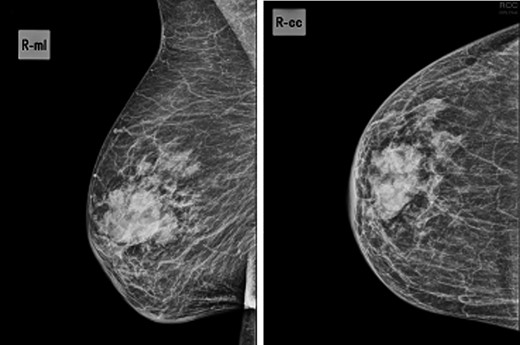
Mammogram—44.5 × 24.5-mm nodular lesion in the transition of the external quadrants of the right breast with lobulated contours and associated skin thickening.
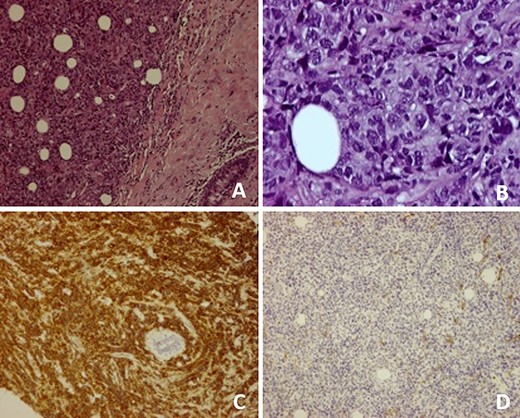
(A) Breast parenchyma with neoplastic infiltration by small- and medium-size lymphoid cells; (B) Cell composition of the neoplasm at higher magnification; (C) CD20 antibody immunostaining present in diffuse large B cell lymphomas; (D) No immunostaining of CD10 antibody, excluding follicular lymphoma.
Regarding management, surgery, radiotherapy, chemotherapy and immunotherapy have been used alone or in combination; however, there is still no consensus on the best approach. Chemotherapy, alone or combined, is the standard treatment [3]. Indeed, surgery does not have any impact on survival or recurrence risk, so it is not recommended. It is only performed for diagnostic purposes or better local control [3, 8]. As far as chemotherapy is concerned, the cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) regimen is the most accepted. Nevertheless, there are still controversies concerning the selection criteria for combined therapy, which is recommended by most authors for high-grade tumors or when there is axillary involvement [3,9]. Central nervous system (CNS) involvement in primary high-grade breast lymphomas has also been described and, accordingly, some authors recommend CNS prophylaxis [10].
Although primary breast lymphoma was initially considered to have a poor prognosis, it is thought to be similar to other lymphomas of the same histological type. The Ann Arbor staging system and the International Prognostic Index are equally used [1, 4]. Five-year survival varies according to case series: 89% for stage I and 50% for stage II. In some reports, age is considered an independent factor for long-term survival [3].
CLINICAL CASE
We present an 88-year-old female patient with past medical history of pulmonary tuberculosis, peripheral venous insufficiency and asthma. There was no personal or family history of cancer, namely breast cancer. The patient was referred due to a lump detected in the right breast. On observation, the patient presented with a palpable mass in the transition of the external quadrants of the right breast with 50 mm in diameter, hard, mobile, non-adherent to the skin or the underlying tissue, as well as a suspicious axillary adenopathy. A mammogram was performed and the lesion was described as a nodular lesion with lobulated contours, with 44.5 × 24.5 mm and associated skin thickening (Fig. 1). On ultrasound, the lesion corresponded to a hypoechoic nodule with lobulated and poorly defined contours as well as with multiple ipsilateral axillary adenopathies, the largest with 20 mm—breast imaging-reporting and data system (BI-RADS 5). A core biopsy of the breast lesion and suspected axillary adenopathy were performed, both consistence with diffuse large B-cell lymphoma, positive for CD20, BCL2, BCL6 and MUM1 (Fig. 2). Nuclear immunostaining was observed in about 90% of neoplastic cells. On staging CT scan, no lesions were found than those already described (Fig. 3). The patient was submitted to R-mini-CHOP (CHOP and Rituximab), completing three cycles, with clinical and radiological response.
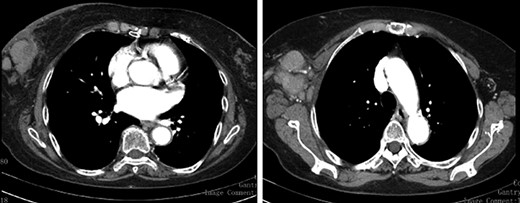
Staging CT Scan—lobulated mass associated with exuberant ipsilateral axillary adenomegalies.
DISCUSSION
This is a rare case of primary breast lymphoma whose clinical presentation has resulted in the appearance of a painless and hard mass associated with suspected axillary adenopathy, clinical findings that are no different from breast carcinoma. In terms of imaging, the characteristics were consistent with the diagnostic hypothesis. The lesion was suspicious, hypoechogenic, with poorly defined contours and was classified as BI-RADS 5. However, after biopsy of the lesion and adenopathy, the histological findings evidenced a diffuse large B-cell lymphoma. There was no previous history of lymphoma and the CT scan study showed no distant lesions, meeting the diagnostic criteria for primary breast lymphoma. In view of this diagnosis, unlike breast carcinoma whose approach is well established and often undergoes surgical management, there are no well-defined therapeutic guidelines due to the rarity of primary breast lymphoma. The treatment must be individualized and multimodal, with surgery reserved for patients who benefit from a better local control.



