-
PDF
- Split View
-
Views
-
Cite
Cite
Tareq Al Saadi, Christopher Sciamanna, Ambar Andrade, Sunil Pauwaa, Gregory Macaluso, Anjali Joshi, Muhyaldeen Dia, William Cotts, Patroklos Pappas, Michael Bresticker, Antone Tatooles, Venoarterial extracorporeal membrane oxygenation use in staged combined heart–kidney transplant, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz408, https://doi.org/10.1093/jscr/rjz408
Close - Share Icon Share
Abstract
Outside of heart and lung transplantation, only few cases have been reported describing venoarterial extracorporeal membrane oxygenation (VA-ECMO) use in solid organ transplantation. We present a case of a staged combined heart–kidney transplant in which VA-ECMO was utilized after a complicated orthotopic heart transplantation to successfully complete the subsequent renal transplantation.
INTRODUCTION
Combined heart–kidney transplant (HKT) has been more frequently used in patients with concurrent heart and kidney failure and has been shown to have survival outcomes that are comparable or superior to those of isolated orthotopic heart transplant (OHT) [1]. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is an option for cardiopulmonary failure. Its use as a bridge to OHT [2] or lung transplant [3] is well established. Outside of heart and lung transplantation only few cases have been reported describing VA-ECMO use in solid organ transplantation. We present a case of a combined HKT in which VA-ECMO was utilized after complicated OHT to successfully complete the subsequent renal transplantation.
CASE PRESENTATION
The patient was a 69-year-old male with past medical history significant for coronary artery disease, ischemic cardiomyopathy, hypertension, stage III chronic kidney disease (CKD) and hypothyroidism. He presented with an acute myocardial infarction unamenable to revascularization and subsequent cardiogenic shock requiring placement of VA-ECMO. Ultimately, a durable left ventricular-assisted device was placed, and due to CKD, he was listed for HKT.
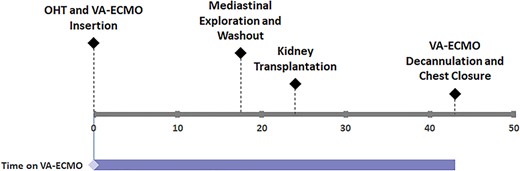
A suitable donor became available and he was taken for staged HKT. OHT surgery was complicated with severe bleeding, hemoptysis and hypoxia requiring massive fluid and blood transfusions. Bronchoscopy revealed diffuse bronchial bleeding that was controlled by positive pressure ventilation. Transesophageal echocardiogram showed preserved biventricular function. Given hypoxia and significant transfusion requirements, there was a concern for the development of allograft right ventricular dysfunction, which could adversely affect the future renal allograft. VA-ECMO was used to protect the right ventricle until renal transplant could be performed. Sternum was left open with plans for primary closure later. On postoperative Day (POD) 1, patient returned to operating room and underwent mediastinal exploration and washout operation in addition to renal transplant. On POD 2, a ramp-down study was performed confirming preserved biventricular function and adequate central venous pressure. VA-ECMO was successfully removed. Patient was discharged home 2 months post-HKT with normal cardiac and renal function. Patient’s surgical timeline is summarized in Fig. 1.
DISCUSSION
ECMO use in patients with cardiopulmonary failure has been persistently growing. It is more frequently reported for high-risk patients in whom ECMO support was previously considered to be contraindicated [4]. In our case, VA-ECMO was used in a patient undergoing a staged combined HKT surgery with OHT complicated by intraoperative bleeding and hypoxia requiring massive blood transfusion. This is one of few reported cases using VA-ECMO in solid organ transplantation outside of heart and lung transplant.
Alveolar hemorrhage is a dreadful and potentially fatal complication of cardiothoracic surgeries. Although ECMO use in the setting of any bleeding is generally discouraged, reports of its use in managing diffuse airway bleed show good outcomes [5]. Given bronchial hemorrhage and the subsequent severe hypoxia in our patient, starting ECMO was deemed essential to provide respiratory support and save the patient’s life.
Right heart failure is a serious complication to heart transplantation that is associated with high morbidity and mortality [6]. Large volume replacement and hypoxia are known risk factors for developing elevated right ventricular pressures and dysfunction, which can ultimately lead to renal congestion and injury. The use of VA-ECMO in the management of post-heart transplant right ventricular failure was associated with good outcomes [6]. Our patient was placed on VA-ECMO to manage his hypoxia and to prevent high pressures that could adversely affect the planned renal allograft. Although the therapeutic use of venovenous ECMO would have been sufficient for managing the bronchial bleed in our patient, VA-ECMO in this case provided additional prophylactic effect on our patient against right heart failure. This approach allowed for adequate hemodynamic stability and successful subsequent renal transplant. This is one of very few reported cases of the therapeutic and prophylactic use of VA-ECMO in this setting. Although more data are needed to draw solid conclusions, this report shows that VA-ECMO use in combined HKT is a reasonable option to manage intraoperative complication and to prevent overload complications.
Funding
There are no sources of funding associated with this work.
Conflict of interest statement
None declared.



