-
PDF
- Split View
-
Views
-
Cite
Cite
Massama Lomdo, Khadija Setti, Mohamed Oukabli, Mountassir Moujahid, Ahmed Bounaim, Gastric schwannoma: a diagnosis that should be known in 2019, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz382, https://doi.org/10.1093/jscr/rjz382
Close - Share Icon Share
Abstract
Gastric schwannoma (GS) is a rare neoplasm of the stomach deriving from Schwann cells of the peripheral nerves in the stomach. It accounts for 0.2% of all gastric tumors and is mostly benign, slow-growing and asymptomatic. Due to its rarity, GS is not widely recognized by clinicians. Preoperatively, GSs are difficult to differentiate from other mesenchymal tumors, such as gastrointestinal stromal tumor (GIST) or leiomyoma, which develop from mesenchymal stem cells. The optimal management of GS is based on the symptoms of the patient, tumor size and histologic grading. Here, we report the case of a GS in a 73-year-old female who underwent a wedge gastric resection following a clinical diagnosis of GIST. A histological and immunohistochemical study was performed excluding the misdiagnosis of GIST. The histomorphological features of the lesion and absence of c-Kit and strong positivity of S100 indicated the diagnosis of GS.
INTRODUCTION
Mesenchymal tumors of the gastrointestinal tract are formed by a group of tumors of spindle cells, which include three major types: gastrointestinal stromal tumors (GIST), smooth muscle tumor and nerve sheath tumor (Schwannomas) [1,2]. Schwannomas can be easily mistaken for a GIST or a leiomyoma, when visualized on upper endoscopy. Schwannomas are slow-growing, homogeneous, mostly benign tumors arising from the Schwann cells of the nerve sheath [3]. They are most commonly found in the cranial vault [4]. This case report highlights the rarity of a schwannoma at the greater curvature of the stomach. The relevant literature is reviewed.
CASE REPORT
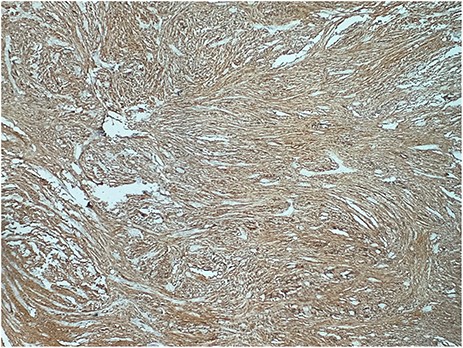
A 73-year-old woman was referred to our general surgery service. Her complain was intermittent gastric discomfort. There was no significant past medical history. There was no history of upper gastrointestinal bleed, or past surgical interventions with no abnormalities found on physical exam. An esophagogastroduodenoscopy (EGD) revealed a submucosal mass in the gastric body. Biopsy of the mass showed no evidence of malignancy. Computerized tomography (CT) scan of the abdomen showed an exophytic solid homogeneous mass along the greater curvature of the stomach and partly projected into the gastric lumen, causing smooth indentation measuring 8 × 8 × 6 cm3 (Fig. 1). The patient’s laboratory results were unremarkable. A presumptive diagnosis of GIST was made. The patient underwent successful wedge resection of the mass through laparotomy and the specimen was sent to pathology. On gross examination, a white intramural, nodular, solid mass measuring 8.7 × 8.8 × 6.7 cm was seen. A cut section revealed whirling trabeculation with a biphasic proliferation of compact hypercellular areas and myxoid hypocellular areas (Fig. 2). Sections from the tumor showed interlacing bundles of spindle cells, which had elongated nuclei, ill-defined cytoplasmic borders and palisading nuclei (Fig. 3). No nuclear atypia was noted. No mitotic activity and no necrosis were identified. There was no lymph node involvement and the surgical margin was negative for tumor cells. A histological diagnosis of a benign mesenchymal tumor was made. Immunohistochemistry (IHC) staining was strongly positive for S-100 (Fig. 4), whereas c-Kit, CD 34; DOG 1; smooth muscle actin (SMA), desmin and AE 1/AE 3 were negative. Hence, a final diagnosis of schwannoma was made. The postoperative period was uneventful and the patient was dismissed from the hospital after 5 days.
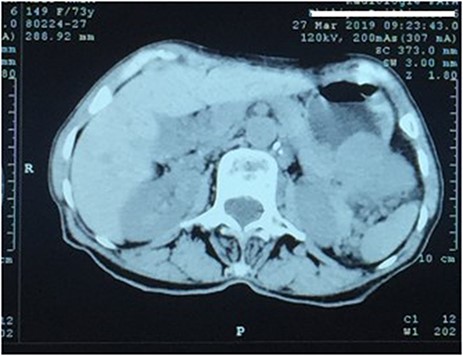
CT scan showing an exophytic mass in the great curvature of stomach wall
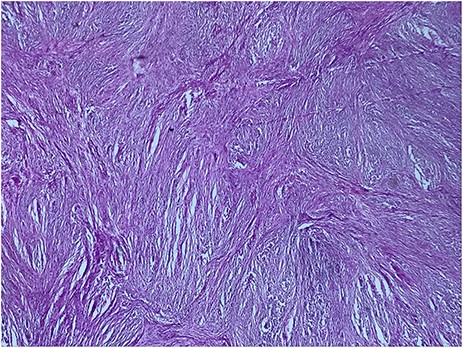
Biphasic proliferation of compact hypercellular areas and myxoid hypocellular areas (HE, G x 25)
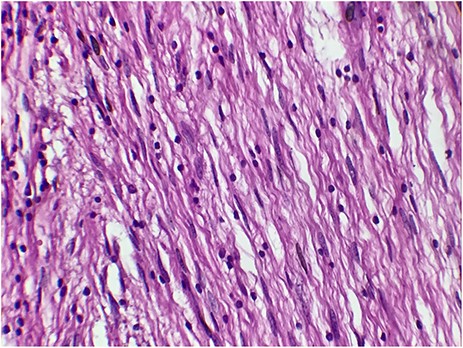
The tumor cells are narrow, elongated and wavy with tapered ends interspersed with collagen fibers (HE, G x 400)
DISCUSSION
Gastrointestinal schwannoma is thought to arise from the nerve plexus of gut wall, in contrast to conventional schwannomas, which can develop anywhere along the peripheral course of nerve. Exceptional location as uterine cervix has been reported in the literature [4]. However, they most commonly occur in the head and neck but they are rare in the GI tract [1,3]. Gastric schwannomas (GSs) are the most common in the GI tract; however, they account for only 0.2% of all gastric tumors and typically involve submucosa and muscularis propria [1].
GS typically grows as a solitary lesion and is commonly located in the body of the stomach with a variable size [4].
According to literature, GS can occur at any age but are most frequently noted in fifth to eight decades with female predominance [2]. These findings are consistent with our case, in which a GS presented in a 73-year-old female.
The symptoms can range from abdominal pain or discomfort to mild dyspepsia, hematemesis palpable abdominal mass and weight loss. The bleeding is thought to occur due to ulceration of the mass from reduced tolerance to gastric acid [1–5]. In 2015, Yang et al. [6] reported a case of gastroduodenal intussusception due to GS, which, to the best of our knowledge, is the only case reported in English literature.
CT is widely used in clinical examinations. GS usually shows a well-defined, oval, submucosal tumor in the stomach with an exophytic or mixed growth pattern and moderate homogeneous enhancement like the case was in our patient as well. GS frequently co-occurs with swelling perigastric lymph nodes [7].
MRI findings of spinal and cranial schwannomas are well described in literature. MRI features of gastrointestinal schwannomas seem to be similar. Most tumors are low to isointense on T1-weighted images and isointense to high intense on T2-weighted images [8].
On endoscopy, GSs appear grossly as elevated submucosal lesions [3]. As shown in this case, endoscopic biopsy may not be adequate for definitive diagnosis because mucosal abnormalities are rarely observed in these submucosal tumors [1,8].
New advances in gastroenterology have increased the role that endoscopy plays in the treatment of GI tumors. Despite these recent developments, tumors that lie within the muscularis propria, or are >3 cm in size require surgical intervention, due to their high risk of perforation [9]. Complete surgical resection is widely considered to be a curative treatment for GS. Our patient’s schwannoma indeed measured >3 cm, making surgical resection the obvious choice.
A helpful histologic clue to the diagnosis of schwannoma is a peritumoral lymphoid cuff, which is rare in GIST [8]. Schwannoma cells have spindle-shaped nuclei and a fascicular arrangement. The diagnosis of schwannoma is based on immunohistochemical positivity for S-100 protein [1,8]. Our case was misdiagnosed as a GIST until these histological and immunohistochemical findings were revealed.
Choi et al. [10] calculated the growth rate based on computed tomography (CT) images of GS patients with a series of follow-ups. The mean doubling time of schwannoma was nearly 5 years. Recurrence of these tumors is rare. A paper published in 2015 by Hong et al. [2] reviewed 137 cases of benign GS and did not identify recurrence or metastasis in any patients during a follow-up period ranging from 1 to 336 months. Another paper published in 2017 by Bao-guang Hu et al. [5] reviewed 221 cases of GS with 211 benign schwannomas and 10 malignant schwannomas and suggested that the follow-up should be conducted over a period of at least 5 years for cases of malignant GS. However, further research is necessary in order to better understand the features of malignant GS.



