-
PDF
- Split View
-
Views
-
Cite
Cite
Schauki Mahmoud, Hosam Salman, Maissam Salami, A rare case report of locally recurrent hemorrhagic duodenal gastrointestinal stromal tumor: therapeutic challenges and review of prognostic indicators for recurrence, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz365, https://doi.org/10.1093/jscr/rjz365
Close - Share Icon Share
Abstract
Gastrointestinal stromal tumor is a rare neoplasm affecting gastrointestinal tract. Duodenal gastrointestinal stromal tumor originating from the fourth segment is considered an extremely rare disease. Surgical challenges arise when managing locally recurrent hemorrhagic duodenal gastrointestinal stromal tumor. A 58-year-old male presented with melena for the last 10 days. Thirty months previously, he had segmental resection of the fourth duodenal portion due to hemorrhagic gastrointestinal stromal tumor. No adjuvant imatinib therapy was administered (low risk for recurrence). The latest investigations showed actively bleeding tumor in the distal third portion of the duodenum, indicating a locally recurrent gastrointestinal stromal tumor. Uneventful emergent limited resection was performed. To the best of our knowledge, this is the first case report describing locally recurrent gastrointestinal stromal tumor in the distal duodenal portion. We will explain the therapeutic challenges and risk stratification and discuss gastrointestinal bleeding as a prognostic indicator for gastrointestinal stromal tumor recurrence.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors originating from the interstitial cells of Cajal in the gastrointestinal (GI) tract. GISTs account for only 1% of all GI neoplasms [1]. GISTs distribute variably in GI tract. Most GISTs locate in the stomach (60%–70%), small intestine (20–30%), colorectum (10%), more rarely in esophagus, appendix, anus and retroperitoneum. Only 1–5% of GISTs occur in the duodenum [2]. Descending part is the most common site of duodenal GIST (51%). Zhen Liu et al found in their recent study and reviewing English literatures about 300 cases of duodenal GISTs. Only 22 patients (8%) had a primary GIST located in the fourth portion of the duodenum [2].
Upper GI bleeding is the most common and the most dangerous clinical manifestation of GISTs (42%) that needs urgent intervention. Other manifestations include abdominal pain (20%) and obstruction (10%). GISTs could be asymptomatic and could be discovered incidentally in approximately 20% [2,3]. GIST cell morphologies are characterized as spindle cell (70%), epithelioid (20%) or mixed (10%). Immunohistochemical staining for CD117 is positive in 98% of GISTs [3]. KIT mutations are found in (70–80%) of GISTs, which affect commonly the expression of exon 11 and rarely exon 9, 13 and 17. Other mutations in platelet-derived growth factor receptor alpha (PDGFRA) (10%) and wild-type (10–15%) are recognized [1]. To the best of our knowledge, this is the first case report describing the management of locally recurrent GIST in the distal part of the duodenum.
CASE REPORT
A 58-year-old male presented to our department with epigastric discomfort and melena for the last 10 days. Thirty months previously, he had a history of melena which was diagnosed as bleeding GIST in the fourth duodenal portion. Segmental resection of the fourth part of the duodenum, end-to-side duodenojejunostomy and feeding jejunostomy were performed. The histopathology study revealed spindle cell type duodenal GIST about 3 × 3cm, mitotic index < 5/50HPF, free surgical margins more than 5 mm and intact tumor pseudocapsule. No adjuvant imatinib treatment was initiated as the tumor was classified as low risk for recurrence according to National Institutes of Health (NIH)-modified (Joensuu) classification [1]. The patient had lost his follow-up. He was admitted lately as emergency to investigate melena. Physical examination revealed pale patient, slight tachycardia and mild hypotension. Abdominal exam showed midline scar with mild tenderness in epigastrium. Rectal digital examination confirmed the melena.
Admission work-up revealed anemia (hemoglobin = 7.8 g/dL, hematocrit = 23%). Emergent upper GI endoscopy showed active bleeding of ulcerated tumor on the third duodenal portion (Fig. 1A and B). Enhanced computed tomography (CT) scan revealed 5 × 4.5 cm hypervascular mass on the previous duodenojejunal anastomosis without metastasis (Fig. 2A and B). Diagnosis of locally recurrent duodenal GIST was suspected. Progressive anemia had developed in spite of transfusion 3 units packed red blood cells, and thus, emergent laparotomy was performed. During laparotomy, we found recurrent mass just on the previous duodenojejunal anastomosis without intraperitoneal or liver implantations (Fig. 3). A careful limited resection of the distal third part of the duodenum with proximal jejunum was carried out (Fig. 4). Side-to-side anastomosis between the second duodenal portion and jejunum was performed (Fig. 5A–C). Feeding jejunostomy tube was inserted. Post-operative course was uneventful. The patient was discharged on the ninth post-operative day. Histopathology report revealed 4 × 3 cm spindle cell type duodenal GIST, mitotic index < 5/50HPF, resection margins free more than 5 mm, intact pseudocapsule with vascular invasion and tumor emboli (Fig. 6A and B). Immunohistochemical staining for CD117 was positive (Fig. 6C). Unfortunately, molecular assessment of the recurrent tumor was unavailable in our institute. Adjuvant imatinib 400 mg/day was administered. To detect any recurrence in the liver or peritoneal cavity during adjuvant imatinib therapy, intensive surveillance with enhanced abdomen and pelvis CT scan every 6 months was recommended [3].
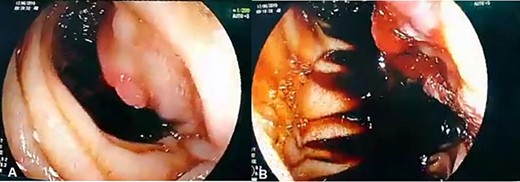
(A, B) Upper GI endoscopy shows centrally ulcerated recurrent tumor in the third portion of the duodenum with active bleeding.
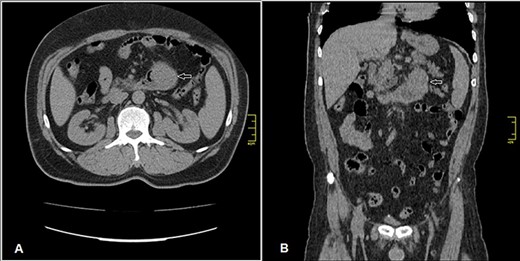
Enhanced CT scan (A) axial section and (B) coronal section: shows well enhanced mass 5 × 4.5 cm (arrow) at previous duodenojejunal anastomosis without intraperitoneal or liver metastasis.
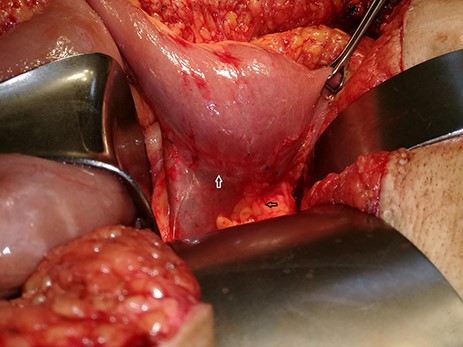
Recurrent tumor on the previous (end-side) duodenojejunal anastomosis (white arrow) and pancreas (black arrow).
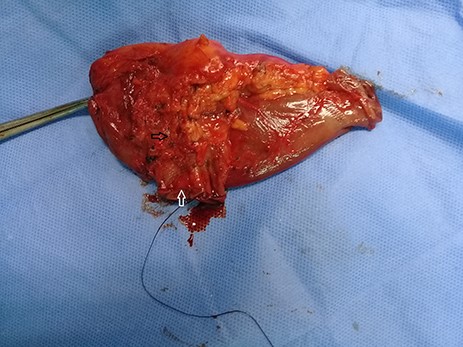
Limited resection of the distal third part of the duodenum (white arrow) with proximal jejunum including the mass (black arrow).
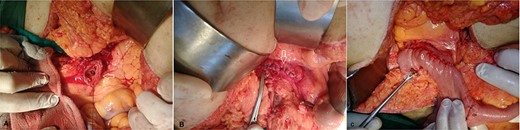
(A) The cut end of the distal third portion of the duodenum (arrow). (B) The closed end of the distal third portion of the duodenum (arrow). (C) Side-to-side anastomosis between the second duodenal portion and jejunum (white arrow), the site of previous feeding jejunostomy tube (black arrow).
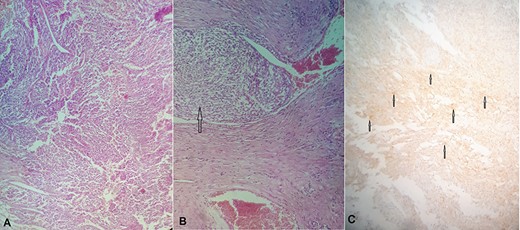
(A) Hematoxylin and eosin (H&E) stain: proliferation of spindle cells of varying cellularity, hyperchromasia and nuclear pleomorphism (GIST spindle cell type). (B) H&E stain: vascular invasion with tumor emboli (arrow). (c): Immunohistochemical staining for CD117 is positive (arrows).
DISCUSSION
Complete resection of the tumor with negative margins, without lymph node excision, is the curative treatment for GISTs. Unlike epithelial neoplasms, submucosal spread and local lymph node involvement are uncommon in GISTs (5%); thus, wide resection margins with routine lymph node dissection are unnecessary [4].
GISTs are considered aggressive neoplasms that have a poor prognosis. Approximately 50% of gastric and nongastric GISTs recur by 5 years after complete tumor resection [1]. Tumor size, mitotic index, site (gastric and nongastric) and tumor rupture are the current four independent prognostic indicators for recurrence [1]. According to NIH-modified risk stratification and Armed Forces Institutes of Pathology (AFIP) criteria, our primary tumor was classified as low risk for recurrence and needed NO adjuvant imatinib treatment [1].
Primary GIST is solitary rather than multiple [5]; furthermore, the incidence of duodenal GIST is very rare [2]. In our case, the newly discovered tumor was found in the same place of the resected primary tumor (duodenojejunal anastomosis), so the possibility of recurrent GIST is so much higher than the occurrence of a new primary tumor.
The recurrence in low risk GISTs is extremely rare. The 5-year disease-free survival rate (DFS) in low risk GIST is about 96%, whereas DFS in moderate and high-risk GISTs is approximately 54% and 20%, respectively [6]; therefore, adjuvant imatinib therapy is preserved to moderate and high-risk GISTs. Unexpected recurrence occurred in our patient.
There is no obvious correlation between histological subtypes and risk of tumor recurrence [4]. The data from the American College of Surgeons Oncology Group (ACOSOG) have demonstrated that KIT mutation status is not an independent predictor of recurrence [1]. The classification of gene mutation often determines post-operative treatment [3].
Gastrointestinal bleeding in GISTs occurs due to mucosal ulceration and tumor invasion into submucosal blood vessels or tumor necrosis. In both circumstances, tumor emboli can be spread into the lumen of the intestine or blood vessels leading subsequently to local recurrence or distant metastasis. Recent researches suggest that GI bleeding could be considered as an independent prognostic indicator for recurrence [3, 7, 8]. These studies announced that GI bleeding has a poor prognosis and should be managed as tumor rupture (high risk). We believe that bleeding in our primary tumor could be the main cause for the local recurrence.
Due to the complicated anatomy and the rarity of duodenal GISTs, therapeutic strategies are still under debate. Histopathology confirmation should be done if neoadjuvant imatinib therapy is considered. In case of unresectable or large tumors, neoadjuvant imatinib for 6–9 months could reduce the size of duodenal GISTs and makes them resectable via lesser operation [1]. Some studies reviewed the surgical options for duodenal GISTs comparing pancreaticoduodenectomy vs. limited resection [4, 9].
Pancreaticoduodenectomy is kept for large GISTs with nearby structure invasion or involving ampulla of vater.
Limited resection includes (i) wedge resection with primary closure, which can be carried out in small tumors affecting the first or second duodenal portions, (ii) segmental duodenectomy with end-to-end or end-to-side duodenojejunostomy, which can be carried out for tumors which are located in the third or fourth duodenal portions (as it was performed to manage our patient’s primary tumor) or side-to-side anastomosis between the second duodenal portion and jejunum (as it was performed in the second surgery) and (iii) side-to-side Roux-en-Y duodenojejunostomy, which can be performed after resection of antimesenteric tumor in the second or third duodenal portions [4].
Limited resection when achieving free surgical margins and avoids tumor rupture is preferable to pancreaticoduodenectomy. Limited resection preserves the continuity of GI tract, contributes a better quality of life and avoids the risks of anastomosis leak and stenosis in pancreaticoduodenectomy [4]. The DFS rate is the same regardless of the kind of surgical procedure [9].
One to three years of adjuvant imatinib 400 mg/day in moderate or high-risk GISTs decreases the risk of recurrence; it is particularly beneficial in patients with the most common gene mutation KIT exon 11 deletions. Imatinib does not delay recurrence in KIT exon 9 mutation, wild-type or PDGFRA-mutant tumors [1, 3]. However, it is acknowledged that one of the limitations of our study is that the gene mutation was not assessed.
In metastatic or recurrent GISTs, imatinib therapy 400 mg/day should be initiated for 6 months followed by surgery for resectable tumors [1]. If tumor size does not reduce in CT follow-up study, dose escalation to 800 mg/day is recommended. In case of tumor progression during imatinib therapy, another tyrosine kinase inhibitor such as sunitinib or regorafenib should be administered [1, 3].
In our case, the lack of interventional angiography facilities [10] made emergent tumor resection necessary.
We believe that current risk stratification schemes may still need some improvement in the future to consider GI bleeding as a new prognostic indicator for GIST recurrence and metastasis.



