-
PDF
- Split View
-
Views
-
Cite
Cite
Masami Takagaki, Hirofumi Midorikawa, Hiroki Yamaguchi, Hiromasa Nakamura, Shinichi Mitsuyama, Tasuku Kadowaki, Yousuke Ueno, Hiroshi Kataoka, Takaki Uchida, Tomoyuki Aoki, Preemptive angioplasty for contralateral leg stenosis following EXCLUDER Iliac Branch Endoprosthesis implantation, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz191, https://doi.org/10.1093/jscr/rjz191
Close - Share Icon Share
Abstract
The GORE EXCLUDER Iliac Branch Endoprosthesis (IBE) device is designed to seal off a common iliac artery (CIA) aneurysm, preserving the internal iliac artery during endovascular aortic repair. We report the case of an 84-year-old man with isolated saccular right CIA aneurysm (35 mm) and a relatively small terminal aorta (24 mm). The IBE device was successfully placed, and intraoperative angiography revealed no leakage or delay. However, postoperative computed tomography revealed marked compression of the contralateral leg by a bridging component. Although his ankle–brachial index was preserved, its acute occlusion was judged highly possible; we decided to perform preemptive angioplasty. The angiography revealed the stenosis only in the left anterior oblique view, and angioplasty was uneventfully performed. The leg was successfully patent at 1-year follow-up. When compression by IBE and bridging component in the terminal aorta is expected, caution should be preserved at intraoperative angiography following the device deployment.
INTRODUCTION
The GORE EXCLUDER Iliac Branch Endoprosthesis (IBE; W. L. Gore & Associates, Flagstaff, AZ) device has been designed to seal off a common iliac artery (CIA) aneurysm, while preserving the internal iliac artery (IIA) during endovascular aortic repair (EVAR). However, its use is precluded by some anatomical limitations, such as insufficient CIA lumen diameter, incompatible IIA or external iliac artery diameter, and insufficient length from the lowest renal artery to the iliac bifurcation [1]. In addition, it necessitates the placement of a larger ipsilateral leg in the terminal aorta using a 23-mm IBE and either a 23 or 27-mm bridging component; therefore, we have to monitor for compression of the contralateral leg in the terminal aorta, particularly for isolated CIA aneurysms. We implanted the IBE device in an 84-year-old man who had an isolated saccular, right CIA aneurysm (35 mm) with relatively small terminal aorta (24 mm), following which preemptive percutaneous angioplasty was needed to correct the compression of the contralateral leg.
CASE REPORT
An 84-year-old man had a recent episode of acute severe pancreatitis because of cholelithiasis. He was also taking oral anticoagulants for paroxysmal atrial fibrillation. While investigating for pancreatitis, computed tomographic angiography incidentally revealed an isolated saccular-type, right CIA aneurysm with a maximum diameter of 35 mm (Fig. 1a). He was referred to us for surgery after the successful management of pancreatitis with laparoscopic cholecystectomy. His ankle–brachial index (ABI) was normal (1.26 on the right side and 1.27 on the left). He was considered as a candidate for EVAR because of his age and risk factors in his recent medical history.
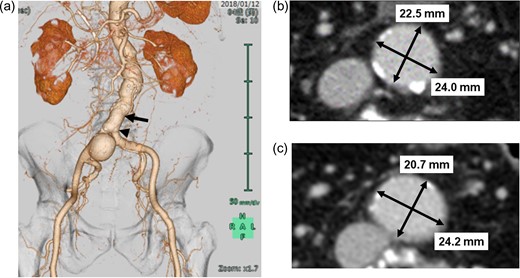
a. Preoperative CT image with three-dimensional reconstruction showing isolated saccular-type, right common iliac artery aneurysm. b. Preoperative axial CT image at the level of black arrow shows relatively narrow abdominal aorta (24.0 × 22.5 cm) approximately 2.5 cm above the level of the terminal aorta. c. Preoperative axial CT image at the level of the black arrow head shows relatively narrow terminal aorta (24.2 × 20.7 cm). CT, computed tomography.
His aneurysm was limited to the right CIA, and his right IIA was widely patent. However, the landing zone in the right CIA was inadequate for applying a ‘bell-bottom’ type device. In cases of conventional EVAR, sacrificing the right IIA with coil embolization is required. Therefore, we decided to preserve the right IIA using the IBE device. The patient’s terminal aorta (24 mm) was marginal to apply this device (Fig. 1b and c).
The device implantation was successful (procedural time, 140 min; contrast load, 120 mL; radiation dose, 275 mGy) with uneventful postoperative course. His intraoperative angiography (anteroposterior view) revealed no delay or stenosis (Video 1). However, his routine postoperative computed tomographic angiography revealed a marked compression of the contralateral leg by the bridging device in the terminal aorta, which was visible only in the left anterior oblique (LAO) view (Fig. 2a). Its axial image seemed similar to a subtotal occlusion (Fig. 2b). Although ABI on the left side was preserved at 1.06, it was lower than the preoperative value (1.27) and that on the right side (1.16). Because we considered its acute occlusion would be the worst scenario for this patient with high possibility of occurrence, we decided to perform preemptive angioplasty for preventing its occlusion. The preoperative angiography found the marked compression only in the LAO view (Video 2). A 10-mm self-expandable stent (LUMINEXX, BARD PERIPHERAL VASCULAR, INC, Tempe, USA) was implanted using balloon angioplasty with improvement (Video 3). Plain computed tomography revealed alleviation of the subtotal occlusion (Fig. 2c). The patient was discharged and has remained asymptomatic during the 1-year follow-up at our clinic. The computed tomographic angiography at 1 year showed successfully patent left leg without any leakage (Fig. 3).
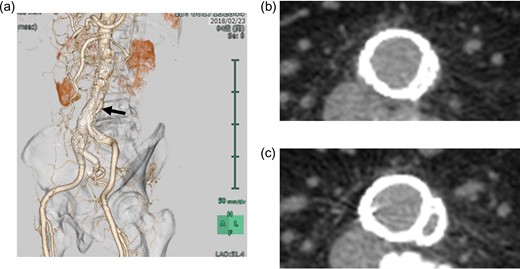
a. Postoperative CT image with three-dimensional reconstruction showing a marked compression of the contralateral leg by the bridging device in the terminal aorta, which is visible only in the left anterior oblique view. b. Postoperative axial CT image at the level of the black arrow shows a marked compression of the contralateral leg in the terminal aorta. c. Postangioplasty axial CT image shows the alleviated compression. CT, computed tomography.
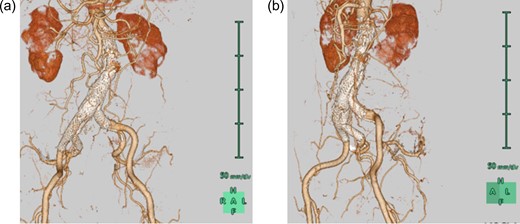
CT images with three-dimensional reconstruction 1 year after endovascular aortic repair. a. Anteroposterior view shows a patent right internal iliac artery without leakage. b. Left anterior oblique view shows the alleviated compression of the contralateral leg in the terminal aorta. CT, computed tomography.
DISCUSSION
The GORE EXCLUDER IBE device has a dedicated IIA branch component with promising initial results [2–4]. This device has been designed to seal off a CIA aneurysm without compromising the antegrade flow of IIA along with standard EXCLUDER EVAR. In our patient, standard EVAR was not feasible without compromising a considerable size of IIA by the coil embolization technique, which is associated with some ischemic complications, such as buttock claudication, erectile dysfunction, colon or intestinal ischemia, and spinal cord ischemia [5–7]. Despite his age, our patient had been spending completely independent lives before his admission for pancreatitis, and rehabilitation was crucial for restoring his independence after a relatively long hospital admission for pancreatitis and aneurysm. Therefore, we considered preserving his IIA with the IBE device.
The proximal side of the IBE device was 23 mm in diameter, and the distal connecting portion of the bridging component was 27 mm in diameter. Therefore, the ipsilateral leg is much bigger than standard EVAR (typically 12–14.5 mm). If the connection between IBE and the bridging component remains in CIA, the terminal aorta can be ≥18 mm in diameter. However, if the connection remains in the terminal aorta, as in our patient, the terminal aorta should be ≥23 mm in diameter. Evidently, our case exhibited a lower limit of the terminal aorta diameter. Among the 46 cases reported by van Sterkenburg et al. [2], one patient had a similar case of symptomatic external iliac limb stenosis; the size of the terminal aorta was not reported. Indeed, caution should have been maintained during the procedure regarding this expected complication.
We were unable to detect this compression stenosis on intraoperative angiography during EVAR, because the stenosis was not visualized at the standard anteroposterior view. The flow to the left leg was not delayed (Video 1). Although the LAO view provided good visualization for the compression stenosis in our case (Video 2), we had not routinely used this view in final angiography to confirm the complete sealing of the aneurysm by the stent graft. In future cases, we plan to perform intraoperative angiography in at least two angles after IBE device implantation. Nevertheless, the development of smaller IBEs and bridging components will be beneficial to patients in whom the implantation of these devices is currently not feasible.
CONCLUSION
Although the EXCLUDER IBE device is a useful tool to preserve ipsilateral IIA, we should have checked for the compression of the contralateral leg in the terminal aorta, particularly in small terminal aorta. However, this complication was easily and safely managed by preemptive angioplasty.
ACKNOWLEDGMENTS
The authors declare that they have no competing interests. The authors would like to thank Enago (www.enago.jp) for the English language review.
CONFLICT OF INTEREST STATEMENT
None Declared



