-
PDF
- Split View
-
Views
-
Cite
Cite
Miya Yoshida, Takuya Ogami, Nora Morgenstern, Litong Du, Myeloid sarcoma resulting in a small bowel obstruction with multiple site involvement including ileum and appendix, Journal of Surgical Case Reports, Volume 2019, Issue 9, September 2019, rjz248, https://doi.org/10.1093/jscr/rjz248
Close - Share Icon Share
ABSTRACT
Myeloid sarcoma (MS) is a rare extra-medullary solid tumor of immature myeloid cells. While it can be an isolated diagnosis, MS is frequently associated with acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic disorders. Although there have been few cases documented that demonstrate the presence of MS in multiple organs at presentation, concomitant involvement of ileum and appendix has never been described. We treated a patient who presented with a small bowel obstruction at the ileum secondary to MS with involvement of the appendix. The patient subsequently underwent a bone marrow biopsy which was negative for evidence of leukemia. He began treatment with induction chemotherapy.
INTRODUCTION
Myeloid sarcoma (MS) is a rare extra-medullary solid tumor of immature myeloid cells [1]. The diagnosis rate is only two per million, with up to 50% presenting without symptoms at the time of diagnosis. While it can be an isolated diagnosis, MS is frequently associated with acute myeloid leukemia (AML), chronic myeloid leukemia and myelodysplastic disorders [2]. It occurs at a rate of 2–8% in patients with AML [3]. Approximately 25% are diagnosed before the occurrence of any hematologic malignancy, 15–35% concomitantly, and 50% are diagnosed after malignancy or as an early manifestation of recurrence [4]. In addition, without treatment, primary MS has been shown to have a high rate of progression to AML, with as many as 80–90% of patients developing AML within 11 months [5]. These tumors may occur in virtually any site in the body; however, the lymph nodes, skin and central nervous system have been noted to be among the most common locations for primary MS to occur [6]. There have been few cases documented that demonstrate the presence of MS in multiple organs at presentation [6, 7]. To our knowledge, however, concomitant involvement of ileum and appendix has never been described. We treated a patient who presented with a small bowel obstruction (SBO) at the ileum secondary to MS with involvement of the appendix.
CASE REPORT
A 47-year-old male presented to the Emergency Department with abdominal pain, nausea, emesis and diarrhea for four days. His last bowel movement was the day prior and he was continuing to pass flatus. On physical examination, his abdomen was soft and nondistended, with tenderness in the epigastrium and left lower quadrant. His vital signs and laboratory values were within normal limits. Computerized topography (CT) scan showed a high-grade SBO with a transition point within distal ileum with asymmetric irregular bowel wall thickening, which was suggestive of a mass (Fig. 1). A moderate amount of ascites was also noted, as well as an appendicolith within the appendix, mildly thickened to 8 mm without any other sign of inflammation (Fig. 2). The patient was admitted to the hospital for initial conservative management, and a cancer work up was initiated for a primary SBO. CT of the chest showed no distant metastases. Tumor markers (carbohydrate antigen 19–9, carcinoembryonic antigen and alpha-fetoprotein) were all within normal limits. Over the next three days, the patient regained bowel function and his pain improved. He opted for outpatient colonoscopy and surgery and was discharged home. Two days later, the patient represented to the ED with abdominal pain, distention and emesis. He had a normal white blood cell count with neutrophilia and lactic acidosis of 3.1. A repeat CT scan of the abdomen and pelvis showed essentially unchanged findings from the prior study. He then was taken to the operating room for exploratory laparotomy. A firm mass was noted 20 cm proximal to the terminal ileum with a mass lesion and an abnormal, firm appendix. We performed a small bowel resection and appendectomy. The patient was subsequently referred to the hematology/oncology department for further treatment. He underwent a bone marrow biopsy which was negative for evidence of leukemia and subsequently began treatment with induction chemotherapy.
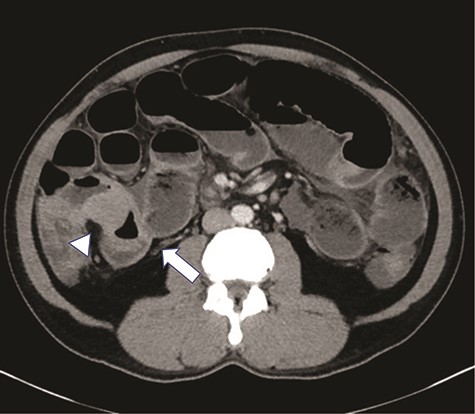
High grade SBO with a transition point within the right lower quadrant in distal ileum (arrow). The bowel loop in this region demonstrates significantly asymmetric irregular wall thickening (arrowhead).
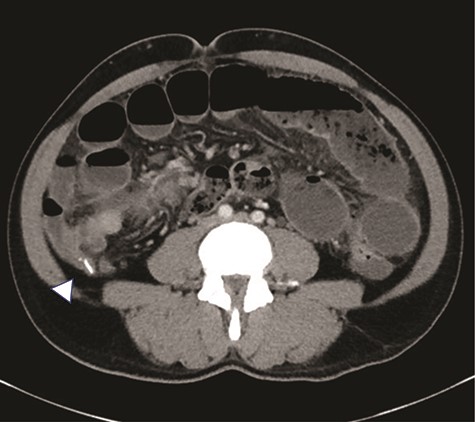
There is an appendicolith noted within the proximal appendix which is mildly thickened measuring up to 8 mm (arrowhead).
DISCUSSION
We performed small bowel resection and appendectomy for a patient who presented with SBO at ileum secondary to MS with involvement of the appendix.
MS has several known names, including granulocytic sarcoma, chloroma and extramedullary myeloblastoma [8]. The name chloroma comes from the presence of myeloperoxidase within granulocytes, which causes the tumor to turn green upon contact to air. Due to its rarity and histologic similarity to non-Hodgkin lymphoma, MS has very high rates of misdiagnosis. In a previous case review, 47% of patients with isolated MS were initially misdiagnosed and subsequently treated inappropriately, thus making proper diagnosis crucial for adequate treatment and prolonged survival [9].
Gastrointestinal MS has been reported to occur at rates from 7 to 11%, with the ileum being the most commonly reported site, possibly due to the high number of Peyer’s patches. In addition, few cases of MS have manifested as appendicitis. Before our patient’s procedure, we had no definitive diagnosis, but an abnormal appendix was noted during the surgery which led us to perform appendectomy. Pathology showed the involvement of appendix. A case of MS involving small intestines, lymph nodes, small bowel mesentery and kidneys was reported previously [6]. However, simultaneous involvement of ileum and appendix has never been reported. Further reports will be needed to define the significance of prognosis.
MS has been treated with surgical resection, local radiation, chemotherapy and stem cell transplantation [10]. Chemotherapy has been shown to decrease progression to AML and median overall survival ranges from 16 to 34 months and 5-year overall survival 17−33% with treatment. A prior study showed an overall survival of 18 months in patients who underwent intensive chemotherapy versus 5 months in those who did not; however, the review did not include patients who underwent surgical intervention followed by chemotherapy. Surgery is typically only performed in cases of hemorrhage, perforation or obstruction. However, concomitant appendectomy can be considered when an abnormality is noted during the surgery for diagnosis and treatment purposes. In addition, it can achieve complete resection, which might result in better prognosis. A staging system of MS has not been described and complete resection might be useful.
In conclusion, we performed small bowel resection with simultaneous appendectomy for a patient who presented with SBO at the ileum secondary to MS with involvement of the appendix followed by chemotherapy. Given the infrequency of cases and thus lack of controlled studies, there has been no agreement on a definitive treatment for primary MS. Although chemotherapy seems to be key, the value of forefront local resection/cytoreductive surgery has not been determined. Simultaneous appendectomy can be considered when abnormality is noted and can achieve complete resection which might result in better prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.



