-
PDF
- Split View
-
Views
-
Cite
Cite
Karishma Seomangal, Paul Neary, Stage IV jejunal adenocarcinoma: a multimodal therapeutic success story, Journal of Surgical Case Reports, Volume 2019, Issue 8, August 2019, rjz239, https://doi.org/10.1093/jscr/rjz239
Close - Share Icon Share
Abstract
Small bowel adenocarcinoma is rare with an incidence of 2.6 in 100 000 patients. Diagnosis is often fortuitous and usually presents late. We present the case of a 36-year-old male who attended the emergency department with worsening abdominal discomfort. A computed tomography scan showed high-grade jejunal obstruction secondary to a jejunal mass suspicious for carcinoma with disseminated peritoneal carcinomatosis and hepatic metastases. Following a conservative approach, his obstruction settled. He commenced on a total of 12 cycles of FOLFOX (folinic acid, fluorouracil and oxaliplatin) and bevacizumab. After re-presenting with intermittent intussusception, a decision for surgical resection was made. On laparoscopy, there was no evidence of hepatic metastases or peritoneal carcinomatosis. A jejunal resection was carried out with an uneventful postoperative period. The patient remains disease free. Despite presenting with an advanced stage, a multimodal approach to these rare tumors may yield surprising and optimistic outcomes.
INTRODUCTION
Adenocarcinoma of the colon is the most common malignancy in the gastrointestinal tract. Despite accounting for 75% of the gastrointestinal tract, the small intestine is not a fertile site for cancer growth [1]. Incidence of small bowel cancers is 2.6 in 100000 persons [2] with adenocarcinoma, carcinoid, lymphoma and mesenchymal tumors like gastrointestinal stromal tumors being the most common [3].
Neoplasms can be linked to Crohn’s disease, celiac disease, familial adenomatous polyposis (FAP), hereditary nonpolyposis colon cancer and Peutz-Jeghers syndrome [3, 4]. Patients with neurofibromatosis also have a higher risk of developing gastrointestinal stromal tumors [3]. Due to the late presentation, patients usually undergo chemotherapy such as FOLFOX [5] (folinic acid, fluorouracil and oxaliplatin) in conjunction with bevacizumab, a monoclonal antibody. Tumor Node and Metastasis (TNM) staging is completed with computed tomography (CT) TAP scan, and confirmatory biopsy with definitive surgery offers the only curative option.
CASE REPORT
Our patient presented with severe epigastric pain and reported similar intermittent episodes for the past 6–7 months. Fecal calprotectin was >200 μg/g (high). An esophagogastroduodenoscopy showed hiatus hernia and an ultrasound abdomen ruled out gallstones. Radiographs showed a dilated loop of jejunum with no pneumoperitoneum.
CT showed high-grade proximal jejunal obstruction secondary to a locally infiltrative jejunal mass (Fig. 1) suspicious for carcinoma. There was disseminated peritoneal carcinomatosis with a small volume of ascites and two hepatic metastases (Figs 2–4). A liver biopsy confirmed metastatic deposit of intestinal origin that was positive for CK20 and CDX2 and negative for CK7 and TTF1. There was a mutation in codon 61 of the NRAS gene with no sign of microsatellite instability. Diagnosis of jejunal adenocarcinoma was made.
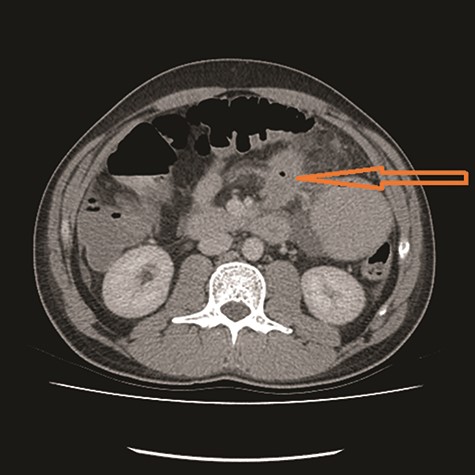
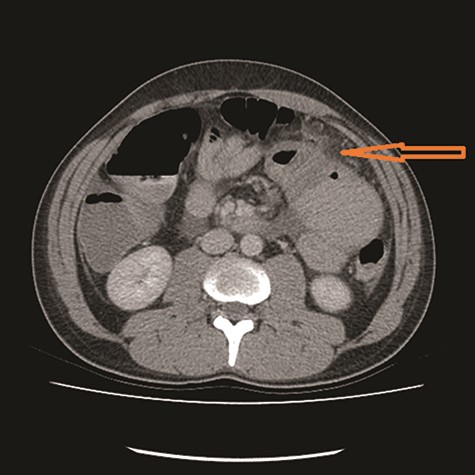
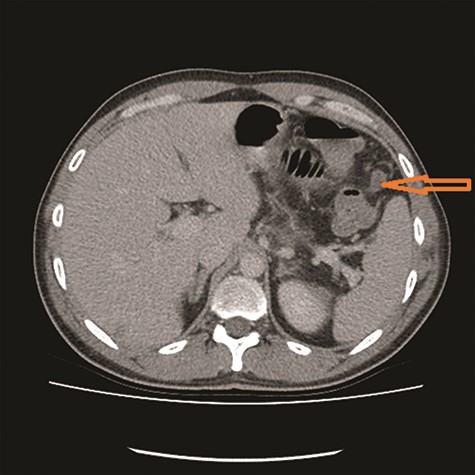
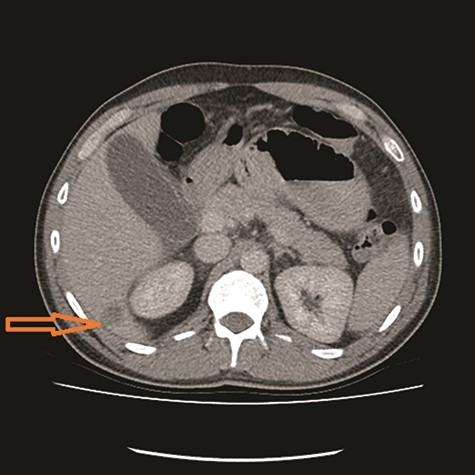
Based on Stage IV presentation, he commenced on FOLFOX and Avastin. After 12 cycles of treatment, his re-staging CT TAP showed decrease in tumor mass, liver metastases and resolution of peritoneal carcinomatosis. He proceeded to elective surgery; at laparoscopy, there was no evidence of peritoneal carcinomatosis or liver metastases. The jejunal mass was resected, and he had an uneventful recovery.
DISCUSSION
Adenocarcinoma of the colon is common while the small intestine is an uncommon site for benign and malignant tumors [1]. When found in small bowel, adenocarcinoma has a predilection for the jejunum, which was the case with our patient and is one of the more common types [1,3]. Carcinoid (2 in 100 000 persons) [6], the other common type, is found in the appendix, ileum and rectum [1]. Gastrointestinal Stromal Tumors (GISTs) (6.8 per million persons) [6] that are most commonly found in the stomach [3] and lymphomas are the other types.
Small bowel neoplasms are associated with familial syndromes like FAP, Peutz-Jeghers syndrome, juvenile polyposis and Cronkhite–Canada syndrome (CCS) [7]. In FAP, an autosomal dominant condition characterized by hundreds to thousands of dysplastic colonic polyps, 80% of affected individuals can have small bowel adenomas as well. These are usually found in the duodenum around the ampulla of Vater [7], and follow-up with capsule endoscopy (CE) or double balloon endoscopy (DBE) is recommended.
Peutz-Jeghers syndrome manifests as mucocutaneous pigmentation and benign gastrointestinal hamartomas. In 90% of affected patients, polyps are found in small bowel although they can be found in stomach and colon as well [4, 7]. They have an increased risk of gastrointestinal and extraintestinal cancers, and 50% of patients will develop a cancer by the age of 60. The cumulative risk of developing small bowel cancer is 13% [7].
Juvenile polyposis is associated with a low risk of gastrointestinal cancer. CCS, with unknown etiology and suspected autoimmune component, is associated with intestinal polyps, pigmentation, diarrhea and protein losing enteropathy. Most of the polyps are found in the small bowel and usually do not become malignant; however, the prevalence of gastrointestinal cancer in CCS is 10% [7].
Small bowel adenocarcinoma is also linked to autoimmune diseases like Crohn’s disease and celiac disease [3, 4]. Patients with neurofibromatosis have an increased risk of developing gastrointestinal tumors [3].
Immunohistochemistry and genetic analyses are valuable and dictate treatment. Most colorectal cancers (and other malignant cancers) show an abnormally high number of epidermal growth factor receptors (EGFRs) [8]. These receptors are found on the surface of cells and upon binding epidermal growth factor promote cell division. Currently, EGFR inhibitors such as cetuximab and panitumumab are in use, and this has been linked to a better prognosis [8]. Tumors that have mutations in KRAS or NRAS gene are deemed ineligible for treatment with EGFR inhibitors [9]. Even wild-type RAS shows a variable response to EGFR inhibitors hypothesized to be due to epigenetic factors. EGFR and HER3 protein biomarkers have been advocated as predictors of better survival [9]. Our patient had a mutation in NRAS and was treated with FOLFOX and bevacizumab, a vascular endothelial growth factor receptor inhibitor.
Presentation is usually asymptomatic. Patients have a vague abdominal pain with nausea and vomiting and may present with anemia, overt gastrointestinal bleeding, intussusception or bowel obstruction emergently [3].
Investigations include barium follow through or small bowel enema (enteroclysis) with evidence that small bowel enemas are more useful in detecting pathology except in Crohn’s disease [7]. The false-negative rate for primary small bowel neoplasms with small bowel enema is much lower at 10% than 80% with barium follow through [7]. CE and DBE can be employed to good advantage. CE may be used initially, while DBE can be used to further evaluate biopsy lesions seen on CE and also retrieve unpassed capsules [10]. DBE is superior to CE with regard to small bowel lesions [10] and facilitates endoscopic dilatation of strictures. CT or magnetic resonance imaging is used to complete the work up and TNM staging.
Neoadjuvant therapy is frequently given. Surgical resection is the only curative option and depends on the location of the lesion. Options include a Whipple’s procedure for lesions in duodenum to right hemicolectomy for terminal ileal lesions. Adjuvant therapy is advocated if there are positive lymph nodes in the resected specimen. Lymph nodes were negative for our patient, and his prognosis is presently guarded with no progression noted.
CONCLUSION
Small bowel adenocarcinoma is uncommon and usually presents late. Despite advanced staging at presentation, the outlook for these patients with multimodal treatments can be very rewarding.
Conflict of interest statement
None declared.
References
- fluorouracil
- abdominal pain
- computed tomography
- carcinoma
- adenocarcinoma
- emergency service, hospital
- intussusception
- laparoscopy
- leucovorin
- diagnosis
- jejunum
- neoplasms
- liver metastases
- small intestine adenocarcinoma
- oxaliplatin
- bevacizumab
- peritoneal carcinomatosis
- excision
- fluorouracil/leucovorin calcium/oxaliplatin
- peritoneal surface malignancy



