-
PDF
- Split View
-
Views
-
Cite
Cite
Vanessa M Baratta, Vadim Kurbatov, Justin M Le Blanc, Brennan Bowker, George Yavorek, Robotic cholecystectomy and cholecystoenteric fistula closure in a female with remote cholangitis, Journal of Surgical Case Reports, Volume 2019, Issue 8, August 2019, rjz231, https://doi.org/10.1093/jscr/rjz231
Close - Share Icon Share
Abstract
Cholecystocolic fistula (CCF), a connection between the gallbladder and neighboring colon, is a rare entity with little consensus as to the optimal surgical management. Existing case reports have described both open and laparoscopic repairs. We describe the first reported case of a successful robotic repair of a CCF in a 50-year-old woman diagnosed with cholangitis 5 years prior to surgery. The patient had a longitudinal follow-up by a single surgeon, allowing for early diagnosis and repair. This case also includes radiographic imaging over 5 years during the index hospitalization and preoperative workup. This allows for a glimpse into the natural pathogenesis of this disease. After robotic surgery, the patient made a complete recovery with no postoperative complications.
INTRODUCTION
A cholecystocolic fistula (CCF) is a two-way connection between the gallbladder and neighboring colon, which often develops as a sequela of chronic inflammation. Patients may be asymptomatic or have vague abdominal pain, making clinical diagnosis elusive. Once identified, a CCF requires surgical repair to prevent progression to sepsis. There is little consensus over the optimal surgical management of this entity. Traditionally, a CCF is managed with open cholecystectomy and fistula closure. With advances in minimally invasive techniques, recent case reports describe successful laparoscopic repairs. Here, we present an asymptomatic female patient with a CCF that was managed with a robotic cholecystectomy and fistula closure—the first of its kind to be reported. This patient also had longitudinal care by a single surgeon, thus we were able to track the natural pathogenesis of her CCF.
Case report
A 70-year-old female with congestive heart failure, atrial fibrillation, and end-stage renal disease presented to the general surgery clinic with a radiographic finding of a CCF. During workup for upper gastrointestinal bleeding, a computed tomography (CT) enterography incidentally demonstrated a fistulous connection between her gallbladder and the proximal transverse colon (Fig. 1). In the clinic, she reported right upper back pain that was unrelated to meals. She had no abdominal tenderness and her liver function tests were within normal limits.
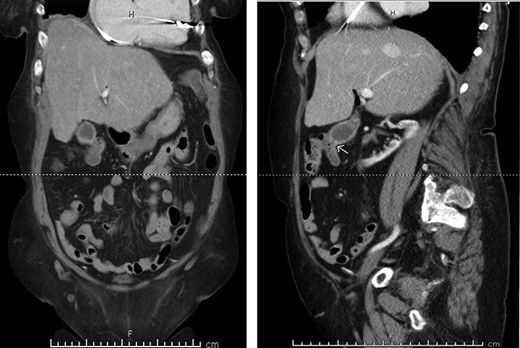
Preoperative CT enterography demonstrating a CCF; (a) coronal view and (b) sagittal view, the white arrow indicates the location of the fistula.
Five years prior to presentation, she was hospitalized for cardiac arrest complicated by septic shock. Laboratory data showed lactic acid of 18.4 mmol/l, a leukocytosis of 41.8 WBC/ul and hyperbilirubinemia. A cholecystostomy tube was placed and a cholangiogram showed a stone-filled gallbladder with cystic duct patency. She showed marked improvement with biliary decompression and the tube was removed after discharge. Because of her multiple comorbidities, she deferred an interval cholecystectomy.
In the clinic, management options for the CCF were addressed and the patient opted for a surgical repair. After medical clearance, a robotic repair of the CCF with cholecystectomy was performed. The da Vinci Xi robotic system was docked to four ports: one in the umbilicus, two in the right lower quadrant and one in the left upper quadrant. The evaluation revealed an inflammatory connection between the gallbladder fundus and the proximal transverse colon. Using sharp dissection and cautery, the fistula was successfully separated. The gallbladder was dissected in a top–down fashion and a critical view of safety was obtained. Locking robotic clips were applied to the cystic duct and cystic artery and the structures were transected. The colotomy was closed in a two-layer running fashion.
The patient made an uneventful recovery and was discharged on the second postoperative day. Histopathological examination of the specimen revealed no evidence of malignancy. The patient was seen in the clinic and had no postoperative complaints.
DISCUSSION
A cholecystoenteric fistula is an uncommon but important complication of gallbladder disease that has historically been managed by open cholecystectomy and fistula closure [1,2]. The three most common types of cholecystoenteric fistulas are cholecystoduodenal (70%), cholecystocolic (20%) and cholecystogastric (10%) [2]. Here, we are the first to describe a robotic repair of a fistulous connection between the gallbladder and the gastrointestinal tract.
Described by Courvoisier as early as 1890, CCF arise from chronic inflammatory conditions in the gallbladder wall, leading to pressure necrosis and erosion into the neighboring tissue [2,3]. The most common etiology is cholecystitis, though patients with inflammatory bowel disease, abdominal trauma and malignancy may be prone to development [3].
In this study, we were able to track the evolution of the patient’s cholecystoenteric fistulae. She was managed by the same surgeon over the 5 years from her initial episode of cholangitis to her eventual cholecystectomy. The initial CT scan taken during her hospitalization shows an inflamed gallbladder abutting the proximal transverse colon (Fig. 2). The CT enterography obtained 5 years later depicts a fistula that developed in this same region (coronal and sagittal views, Fig. 1). Intraoperatively, the fistula was also found at this location. It is difficult to ascertain when the fistula developed as the patient may have been asymptomatic for years.
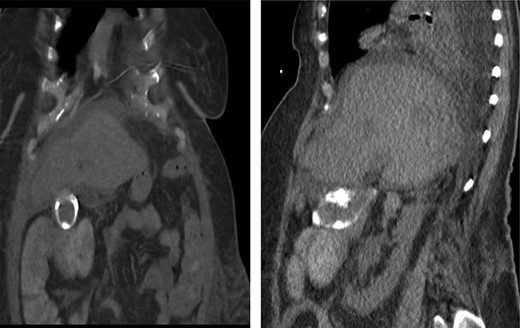
CT scan of the same patient during her hospitalization for cholangitis 5 years prior; of note, there is close proximity between the gallbladder and proximal transverse colon; (a) coronal view (b) sagittal view.
Because many patients with CCF are asymptomatic, it may be difficult to preoperatively diagnose the condition [4,5]. In the literature, a range of reported symptoms include diarrhea, right upper quadrant pain, jaundice and/or fever [1–4]. As in this patient, it is beneficial to repair the fistula once it is identified in order to prevent progression to sepsis. The fistulae may also be associated with gallbladder malignancy, mandating surgical investigation [4].
Whether identified on preoperative imaging or intraoperatively, CCFs have been traditionally managed with open repair. With advancements in laparoscopy, the past few decades have found a growth of laparoscopic management of these fistulas [6–9]. In a single center in China, 29 patients with cholecystoenteric fistula from 2004–2014 were laparoscopically treated with a 17.2% conversion rate to open surgery [9]. Numerous case reports within the past decade have described successful laparoscopic management of cholecystoenteric fistulae [6–9].
Nevertheless, some argue that an open repair remains the best management of CCF. A multicenter study showed that there is a high rate of early conversion to open procedures exceeding 50% [6]. Laparoscopy may put the patient at risk for iatrogenic colon perforation or avulsion of the fistula. In addition, laparoscopic repair requires advanced intracorporeal suturing and often longer operating times for dissection.
Overall, we describe the first successful robotic repair of a cholecystoenteric fistula. In the bevy of literature both in favor and against the minimally invasive repair of cholecystoenteric fistula, this case report suggests that there are several advantages for minimally invasive repair. The robot provided a great degree of precision and made it easier to distinguish and separate the chronic inflammatory tissue between the gallbladder and the colon. In spite of the dense adhesions surrounding the chronically inflamed gallbladder, a top–down dissection facilitated a safe dissection of the gallbladder from the liver bed. More surgeons are becoming robotically trained, and complex hepatobiliary diseases, such as CCF repair, will become incorporated into their surgical repertoire.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES



