-
PDF
- Split View
-
Views
-
Cite
Cite
Masashi Ishikawa, Hiroyuki Chou, Naoto Imamura, Yumeta Shimazu, Kazuo Ono, Malignant triton tumor of the left thoracic cavity: a case report, Journal of Surgical Case Reports, Volume 2019, Issue 8, August 2019, rjz246, https://doi.org/10.1093/jscr/rjz246
Close - Share Icon Share
Abstract
Malignant triton tumor (MTT) is a rare subtype of malignant peripheral nerve sheath tumors with rhabdomyoblastic differentiation. Although the condition may manifest sporadically, it typically affects adult patients with neurofibromatosis type 1. In this article, an extremely rare case of MTT with chest wall origin, which expanded into the left thoracic cavity, is reported. A 64-year-old male was admitted to the institution with sudden shortness of breath. Radiological examination revealed a large mass with massive pleural effusion occupying the patient’s left hemithorax. A percutaneous needle biopsy was performed and the patient underwent subtotal tumor resection with left pleuropneumonectomy. Immunohistochemical study of postsurgical pathologic specimens confirmed the diagnosis of MTT. Despite extensive surgical removal, tumor recurrence was reported soon after resection, leading to patient’s death 20 days after surgery due to acute respiratory failure. Investigation of rare MTT cases is necessary for understanding this condition.
INTRODUCTION
Malignant triton tumor (MTT) is a rare subtype of malignant peripheral nerve sheath tumors (MPNSTs) showing rhabdomyosarcomatous differentiation. MTTs account for < 10% of MPNSTs and exhibit an aggressive course of disease. Although the condition typically manifests as part of neurofibromatosis type 1 (NF-1), it can arise in any part of the body with peripheral nerves. The prognosis is generally poor, and an optimal treatment strategy has yet to be established.
In this article, a sporadic case of MTT with chest wall origin, which invaded into the left thoracic cavity, is presented. Investigation of the clinicopathological characteristics of this rare tumor may assist clinicians in appropriately managing this disease and consequently improving prognosis.
CASE REPORT
A 64-year-old male with a 92-pack/year smoking history was admitted to the emergency department of the institution with sudden shortness of breath. Chest X-ray and computed tomography on admission revealed the presence of massive left pleural effusion and a large tumor (diameter: 13 cm) occupying the patient’s left thoracic cavity (Fig. 1). Pleural effusion cytology and a bronchofiberscopic study failed to show any evidence of disease. Under local anesthesia, percutaneous needle biopsy performed through the left thoracic wall revealed the presence of a sarcomatous lesion (Fig. 1) and positive immunohistochemical staining for CD56 and desmin. Based on these findings, the diagnosis of MTT was considered. There was no evidence of distant metastases.
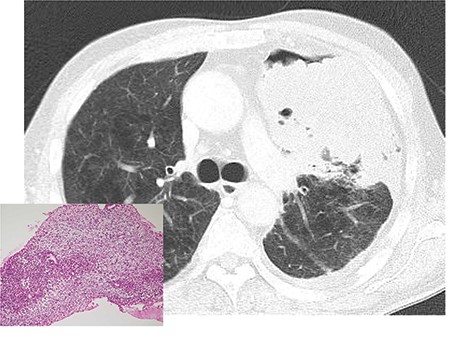
Chest computed tomography after tube thoracostomy. A large tumor with irregular margins occupied the left thoracic cavity. Percutaneous needle biopsy showed a malignant sarcomatoid neoplasm, which was suggestive of malignant triton tumor (left bottom).
The patient underwent surgical removal of the tumor approximately 1 month after admission to the hospital. Conventional thoracotomy was performed at the left fifth left intercostal space, with the patient in the right decubitus position. The tumor occupying the left hemithorax was gelatinous soft and had arisen from the anterior thoracic wall around the second left intercostal space. Moreover, numerous pleural disseminated nodules were identified throughout the left thoracic cavity. A subtotal tumor resection with extrapleural pneumonectomy (without diaphragmatic pleura) was performed due to the invasion of the tumor into the left lung. Broad chest wall removal was deemed excessively aggressive. Thus, the tumor of the anterior chest wall was not removed, and postsurgical radiotherapy was planned. Following tumor removal, the left thoracic cavity was irrigated with distilled water containing cisplatin (100 mg in total). The total operation time was 280 minutes, and the total bleeding volume was approximately 1640 ml.
Macroscopically, the resected tumor was 14 × 8.5 cm in size, solid and gelatinous soft with broad necrosis. Moreover, it had widely invaded the subpleural fat tissue and left upper lobe parenchyma (Fig. 2). Microscopically, short spindle tumor cells embedded in the myxoid stroma showed patternless proliferation, with tumor-free margins displaying broad necrosis. Immunohistochemical examination of tumor cells was positive for vimentin, CD56, CD57, desmin and myoglobin. In contrast, it was negative for pancytokeratin, epithelioid membrane antigen, cytokeratin 5/6, cytokeratin 7, thyroid transcription factor 1, S-100, neuron-specific enolase, CD34, c-KIT, bcl-2, calretinin, alpha-smooth muscle actin, muscle-specific actin, h-caldesmon and alpha-fetoprotein (Fig. 3). The diagnosis of MTT was confirmed based on the evidence of bidirectional differentiation into neurogenic and myogenic components.
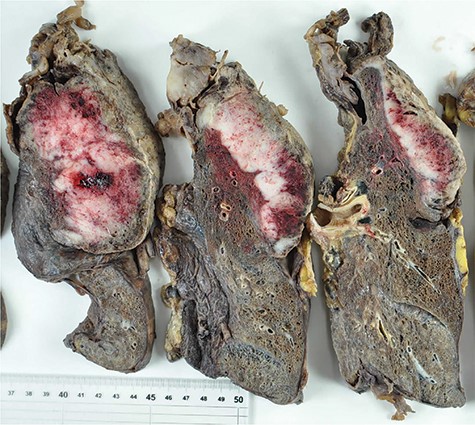
Cut surface of the resected tumor and left lung showing extensive tumor necrosis and invasion into the left upper lobe parenchyma.
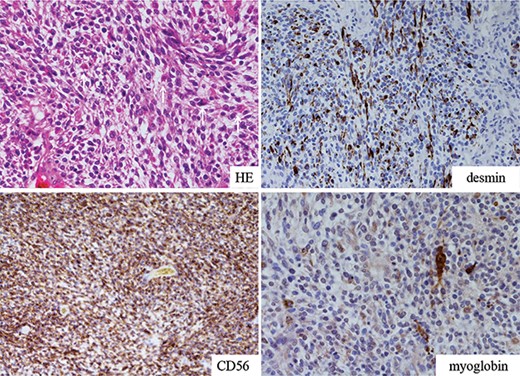
Microscopic view of the resected tumor. Short spindle-shaped sarcomatoid tumor cells with myxoid background showing patternless proliferation revealed positive staining for desmin, myoglobin and CD56. Larger cells with eosinophilic cytoplasm represented rhabdomyoblastic differentiation (white arrows).
The initial postoperative clinical course was unremarkable. However, hypoxemia deteriorated 12 days after operation, and chest computed tomography revealed the presence of fibrotic shadows in the right lung and multiple nodules in the left thoracic cavity. Lymphangitis and pleuritis carcinomatosa were suspected, suggesting early recurrence of disease. Administration of steroidal pulse therapy and intravenous cyclophosphamide was ineffective. Acute respiratory failure progressed, and the patient expired 20 days after surgery.
DISCUSSION
In 1932, Masson described the first case of MTT as a neurogenic tumor accompanied by rhabdomyoblasts in a patient with NF-1 [1]. Approximately two-thirds of MTT cases are associated with NF-1 [2–4], whereas the remaining cases arise in postradiotherapy patients or sporadically [5]. Of note, the average age of patients with sporadic MTT is greater than that of NF-1 patients (i.e. 30–40 years), with a similar distribution according to gender.
MTT occurs at any site of the body with peripheral nerves. However, those of thoracic wall origin are relatively rare. Thus far, only two cases of chest wall MTT have been reported [2, 6]. Other reported cases of thoracic MTT originated from the mediastinum or lung parenchyma [7–9]. Most of these cases showed residual disease or recurrence of tumor after surgery, due to the aggressive spreading of tumor cells into the thoracic cavity, rendering the management of this disease challenging.
The diagnosis of MTT is based on pathological examination. The basic histological presentation is atypical spindle cell proliferation embedded in an abundant myxoid stroma. Demonstrating the bigeminal origin is the mainstay for accurate diagnosis. Intriguingly, in the present case, needle biopsy led to the definitive diagnosis of MTT.
An optimal treatment strategy for MTT has yet to be established. Although radical tumor excision with adequate margins is the intervention of choice, postsurgical local recurrence or distant metastases are common. In the present case, subtotal resection of the tumor was ineffective due to the extensive spreading of the tumor. Although the use of antitumor agents (e.g. cisplatin, etoposide, ifosfamide, doxorubicin or dacarbazine) has been proposed, an effective chemotherapeutic regimen has not been defined [4, 6, 7]. Adjuvant radiotherapy should also be administered to prevent local recurrence, although sensitivity to radiation or the optimal total dose has not yet been determined [3, 5, 8]. Reports of successfully treated MTT cases are valuable for establishing an effective treatment modality.
The prognosis of MTT is dismal. The 5-year survival rate for MTT is < 20%, and most MTT cases have been associated with poor patient outcome [2–6]. As previously reported, subtotal cytoreduction of MTT is not linked to a prognostic advantage. Prognosis depends on the location and grade of the tumor and completeness of resection.
In conclusion, this was an extremely rare case of MTT occupying the left hemithorax. Histological diagnosis was successfully reached through a needle biopsy. Despite the gross subtotal resection, aggressive tumor recurrence was observed shortly after surgery. Thorough investigation of MTT cases is necessary to determine the optimal treatment modality for this condition.



