-
PDF
- Split View
-
Views
-
Cite
Cite
Chairat Burusapat, Nutthadon wongprakob, Rapeepat Sapruangthong, Chatchai Pruksapong, Viriya Kaewkangsadan, Kittisak Wongchansom, Kantang Satayasoontorn, Primary angiosarcoma of the breast presenting with a benign vascular skin-like lesion and expanding hematoma: a case report of an extremely rare tumor, Journal of Surgical Case Reports, Volume 2019, Issue 7, July 2019, rjz223, https://doi.org/10.1093/jscr/rjz223
Close - Share Icon Share
Abstract
Primary breast angiosarcoma is an extremely rare tumor type (0.05% of primary breast cancers) for which diagnosis can be difficult. They arise within the breast parenchyma and typically present as a breast mass. Here, we present the case of a 30-year-old female with spontaneous hypervascular skin on her right breast with progressive enlargement presenting as an expanding hematoma. A chest computed tomography revealed a hypervascular mass in the right breast abutting the pectoralis muscle and cystic changes within the mass. A core needle biopsy revealed an angiosarcoma. In this case study, we report a patient who, with no history of any known risk factors, presented with a benign vascular skin lesion as the first sign of angiosarcoma followed by an expanding hematoma, which is an extremely rare manifestation of the disease. Microscopic examination demonstrated a low-grade angiosarcoma on the skin, while a high-grade angiosarcoma was found in the breast parenchyma.
INTRODUCTION
Angiosarcoma of the breast is a rare malignancy that is divided into primary and secondary types according to the cause of the tumors. Primary breast angiosarcoma is defined as a malignant proliferation with endothelial differentiation [1]. It is an extremely rare tumor type (0.05% of primary breast cancers), for which diagnosis may be difficult [2]. Here we report the unusual presentation of this tumor type, our treatment and the short-term result.
CASE REPORT
Approximately 2 years ago, a 30-year-old female, with no history of breast surgery, irradiation or pregnancy, presented with a spontaneous, non-symptomatic, hypervascular skin lesion measuring 2 × 2 cm on her right breast. The skin lesion had been gradually increasing in diameter, without any discernable mass or bleeding. Her right breast had undergone rapid enlargement 3 months previously after she had fallen from a motorcycle. A conservative treatment was initially performed using cold compression and analgesic drugs. However, her right breast continued to show progressive enlargement. A physical examination revealed a hypervascular skin lesion measuring 5 × 5 cm on the right breast near the areolar and the large mass was also detected (Fig. 1). The right axillary lymph node was found to measure 1 cm after palpation. A mammogram revealed a large circumscribed hyperechoic mass occupying nearly entire right breast and exhibited multiple internal cystic areas, hypoechoic masses and internal vascularity (Fig. 2). A chest CT showed a hypervascular mass in the right breast measuring 13.7 cm that abutted the pectoralis muscle as well as cystic changes measuring 5.3 cm within the mass (Fig. 3). A metastasis workup was unremarkable, and a fine needle aspiration (FNA) was unable to provide a definitive diagnosis. Therefore, a core needle biopsy was performed on the largest hypoechoic nodule and revealed primary breast angiosarcoma. A right-side total mastectomy was performed with 3 cm margins, pectoralis major muscle resection and axillary lymphadenopathy dissection (Fig. 4). Frozen sections exhibited no malignancies at the margins. A split-thickness skin graft (STSG) was performed, covered and healed well (Fig. 5). The pathology report found poorly differentiated, involving breast parenchyma and the dermis, a tumor measuring 16 cm at its widest axis, a mitotic index of 23/10 HPF, and focal tumor necrosis at 10% of the total tumor volume. All 13 axillary lymph nodes were negative for malignancy (Figs 6–8). No post-operative complications occurred, and no postoperative adjuvants were given.
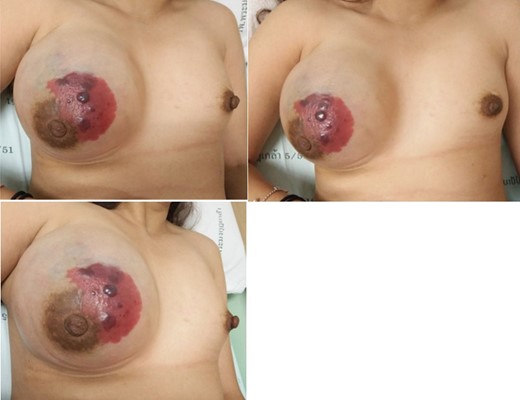
A physical examination revealed a hypervascular skin lesion measuring 5 × 5 cm on the right breast near the areolar and a large mass in the right breast.
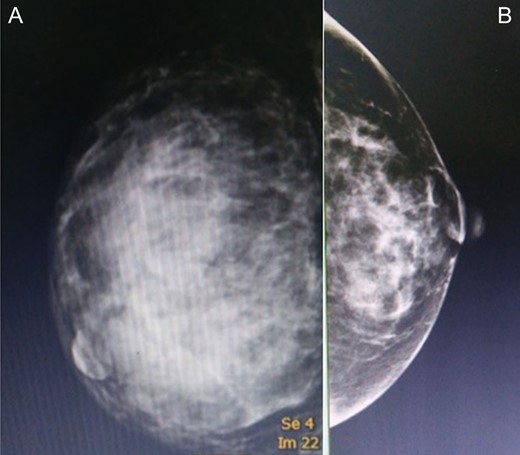
A mammogram of the breasts revealed a large circumscribed hyperechoic mass occupying nearly the entirety of the right breast and comprised multiple internal cystic areas, hypoechoic masses and internal vascularity (A), comparison with the left breast (B).
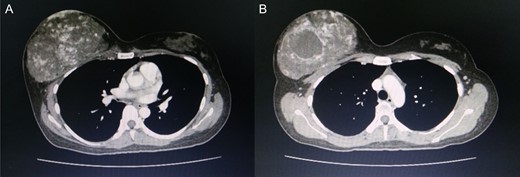
Computed tomography of the chest revealed a hypervascular mass in the right breast measuring 13.7 cm abutting the pectoralis muscle (A) cystic change measuring 5.3 cm in the mass is visible (B).
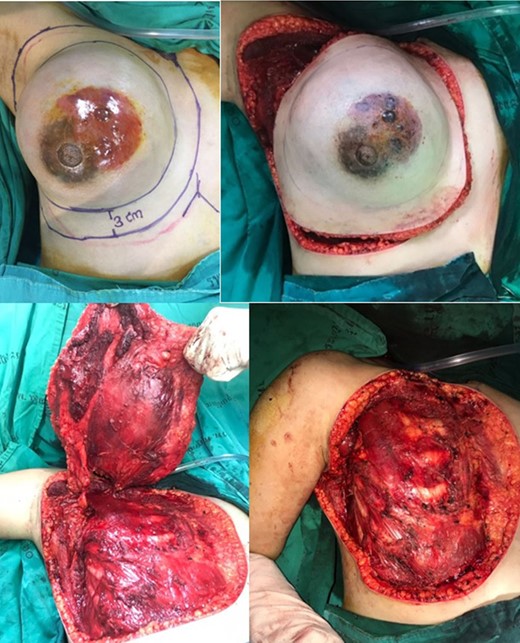
A right-side mastectomy with 3-cm margins with pectoralis major resection and axillary lymphadenectomy dissection was performed.
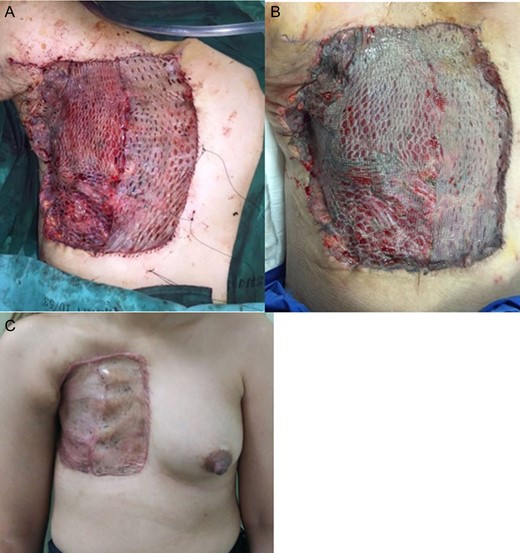
Coverage of the STSG immediately postoperative (A), 1-week postoperative (B), 2-months postoperative (C).
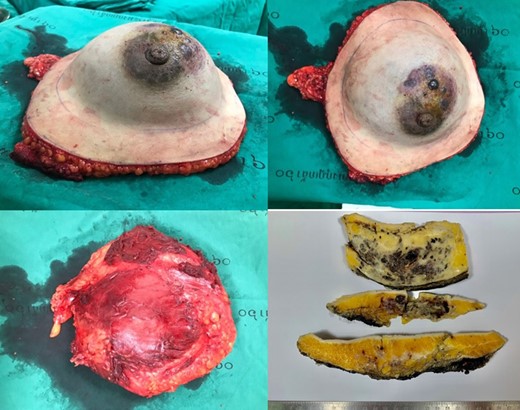
Surgical specimen measuring 23.0 × 20.0 × 9.0 cm and weighing 560 gm. with an axillary tail attached measuring 6.0 × 3.0 × 2.0 cm. The overlying 21.0 × 19.0 cm white/tan skin ellipse shows multiple skin nodules measuring 0.5–0.8 cm. The specimen was serially sectioned and revealed spongy hemorrhagic appearance with ill-defined borders, measuring 16.0 × 12.0 × 4.0 cm and located at the midline, under the nipple and close to the deep margin. The lesion was approximately located at 6.0, 6.5, 6.0 and 7.0 cm away from the superior, inferior, medial and lateral radial margins, respectively.
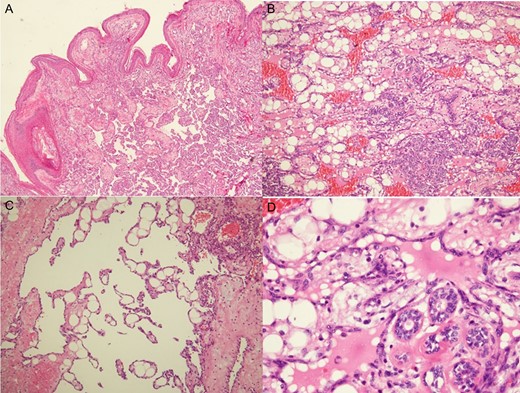
Histologic examination revealed low-grade angiosarcomas on sections of the skin nodules (A) and a mammary mass with lesions varying from well to poorly differentiated vascular neoplasms. The well-differentiated component comprised distinct anastomosing vascular channels that dissected the adipose tissue and lobular stroma (B). Neoplastic vessels had variably dilated or angulated lumina (C). The nuclei of the endothelial lining of the neoplastic vessels was prominent and hyperchromatic, but mitotic activity was infrequent, and there was typically no endothelial multilayering (D).
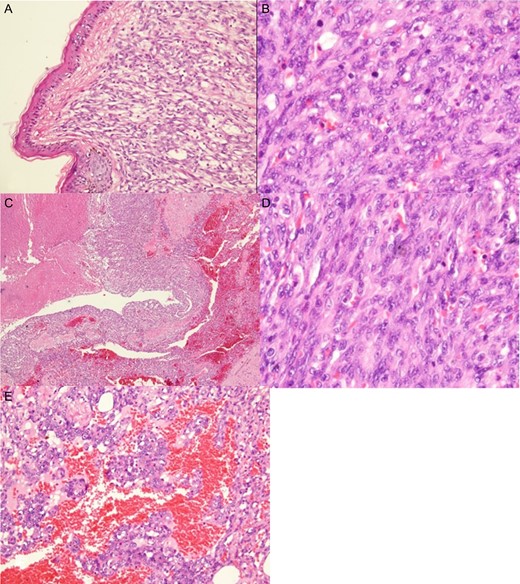
Histologic examination showed a high-grade angiosarcoma in the breast parenchymal section. The poorly differentiated component comprised easily recognizable malignant cells forming infiltrative solid cellular areas (A), with spindled-to-epithelioid pleomorphic hyperchromatic nuclei and abundant eosinophilic cytoplasm intermingled with anastomosing vascular channels (B). In addition, there were blood lakes, necrotic foci (C) and numerous mitoses (D), as well as endothelial multilayering and papillae (E) identifiable in the poorly differentiated areas.
DISCUSSION
Mammary angiosarcoma is defined as a malignant proliferation with endothelial differentiation and is classified into two types. Primary breast angiosarcoma typically occurs in young women with no history of mammary carcinomas [1]. However, secondary angiosarcoma is induced by radiation or chronic lymphedema and is more common than primary angiosarcoma.
In contrast to secondary angiosarcoma, the natural history of the primary type is only partially understood. It is believed that they arise within the breast parenchyma and then infiltrate the overlying subcutaneous tissue and skin [3]. Therefore, it typically presents as a breast mass and not a skin lesion.
Primary breast angiosarcoma occurs more frequently in young women with no previous history of cancer or other risk factors [2]. Up to 12% of primary angiosarcomas are diagnosed during pregnancy, or shortly thereafter, indicating hormonal involvement in its etiology. Rapidly growing breast lumps, which are typically not tender during palpation, are the most prominent physical examination features [3]. Other presenting signs have been described, which include purplish discolorations, eczematous rashes, and diffuse breast swelling [4].
Primary breast angiosarcomas that present with skin lesions are unusual and can lead to misdiagnoses. Combemale et al. reported two patients with primary breast angiosarcomas involving skin lesions. The first patient in that study presented with a superficial, acquired angioma and the second with an indolent, superficial hematoma [5].
Our patient had no known history of any risk factors for angiosarcoma. A benign vascular skin lesion was the first sign, followed by an expanding hematoma; an extremely rare presentation. A microscopic examination revealed low-grade angiosarcoma on the skin with a high-grade angiosarcoma in the breast parenchyma. Previous study reported the angiosarcomas arising from vascular malformations. However, all of the patients in that study were aged between 60 and 70 years and the main presenting symptom was an enlarging, deep-seated mass. Furthermore, three of the tumors were located in the lower extremities, one in the retroperitoneum, and one in the parotid region [6].
Mammograms and ultrasounds do not provide pathognomonic signs for angiosarcomas, hence when masses are found, they may be mistakenly labeled as benign [4]. MRI is more sensitive in characterizing breast lesions, but there is no distinctive feature for angiosarcomas [2].
False negative rates of 40% of FNA have been reported [2, 3]. Therefore, primary angiosarcoma diagnosis is typically confirmed by core biopsy [3]. An excessive bleeding after FNA or biopsy may be evidence of this highly vascular tumor [4].
Histological grading was previously considered predictive of prognosis. However, recent data have indicated that angiosarcoma grade has no prognostic value. Low-grade lesions can metastasize, and second locations can occur in the lungs, liver, bone and skin.
Presently, there is no consensus treatment for primary breast angiosarcoma and current recommendations are derived from retrospective case reviews or extrapolated from non-breast soft tissue sarcoma studies [1]. Surgery remains the primary curative treatment option. Pre-operative radiotherapy is not recommended. Furthermore, adjuvant radiotherapy after surgery, while allowing for better local control, does not affect overall survival rates. However, surgery followed by chemotherapy has shown marginal efficacy in cases of metastatic disease or recurrence [3]. The overall 5-year survival rate after surgery and chemoradiation is 70–79% for grade I and II lesions and 15–30% for grade III lesions [7].
We discussed the surgical procedure, postoperative treatment and the option for breast reconstruction surgery with the patient. She declined the immediate reconstruction and accepted the disfigurement. Following tumor removal, the axillary region was exposed and several significantly enlarged axillary lymph nodes were found. We decided to perform a standard axillary lymph node dissection for complete pathologic staging. Long-term follow-up of primary angiosarcoma patients is important.
CONFLICT OF INTEREST STATEMENT
None declared.



