-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel E Kaczynski, Daniel Fegely, Matthew Nier, Neysa Valentin-Capeles, Jackie Battista, Primary non-urothelial squamous cell carcinoma masquerading as a perforated gastric ulcer: a case report, Journal of Surgical Case Reports, Volume 2019, Issue 7, July 2019, rjz216, https://doi.org/10.1093/jscr/rjz216
Close - Share Icon Share
Abstract
We present an 84-year-old female patient with a very rare form of primary non-urothelial squamous cell carcinoma of the bladder, found incidentally during emergency exploratory laparotomy for a perforated pre-pyloric gastric ulcer. The bladder tumor was positive for CK5/6, CK903, and thrombomodulin biomarkers, as well as for high-risk HPV (16, 18, and 31). Based on a literature review of non-urothelial bladder cancers, specifically non-bilharzial squamous cell carcinoma, we believe our patient had a very rare form of primary non-urothelial squamous cell carcinoma of the bladder. The presence of these tumor markers and the lack of clinical evidence to suggest another primary origin, such as anus, rectum, cervix, or uterus, support this conclusion. This case provides an interesting example of a very rare incidental finding during an emergent procedure.
INTRODUCTION
We present a case of an 84-year old woman with a rare form of primary non-urothelial squamous cell carcinoma of the bladder found incidentally during emergency exploratory laparotomy for a perforated pre-pyloric gastric ulcer.
CASE REPORT
Our patient presented with 5 hours of severe epigastric abdominal pain and anorexia without nausea, emesis, chest pain, shortness of breath, and changes in bowel habits or flatus. She had a history of gastrointestinal bleeding of undefined origin requiring transfusion 2 years previously, and disclosed copious ingestion of aspirin for ongoing abdominal pain.
She was a former smoker with past medical history of hypertension, cerebrovascular event, hypothyroidism, and hyperlipidemia, and esophagogastroduodenoscopy and colonoscopy for gastrointestinal bleed, without past surgical history. On physical exam, she was normothermic, tachycardic, hypotensive and saturating 100% on room air. She had a distended, diffusely tender abdomen with guarding and rebound tenderness. Laboratory results were notable for leukocytosis with a left shift, chronic anemia, and acute kidney injury. Computed tomography of the chest, abdomen, and pelvis showed esophageal thickening, free air under the diaphragm, duodenal inflammation, generalized mesenteric fat stranding, free fluid in the abdomen and pelvis, and a thickened and inflamed bladder wall with irregular foci of hyperintensity within the bladder lumen and a left hydroureteronephrosis (Figs 1–3).
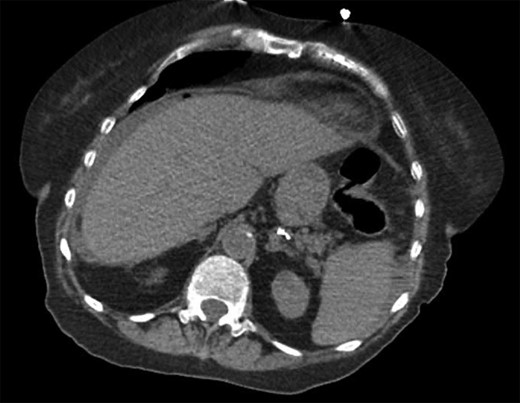
Transverse computed tomography showing intra-peritoneal free air under the diaphragm.
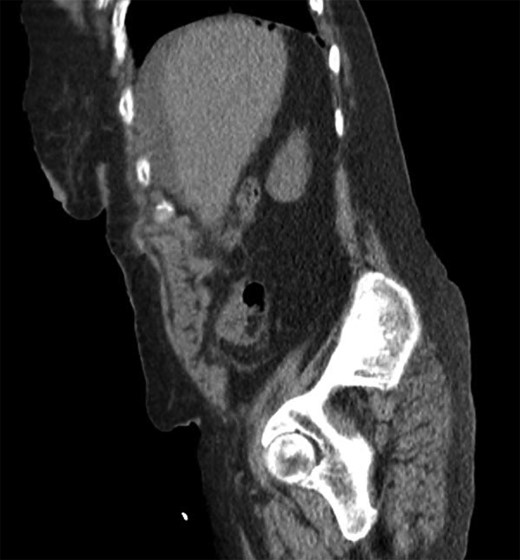
Sagittal computed tomography showing intra-peritoneal free air under the diaphragm.
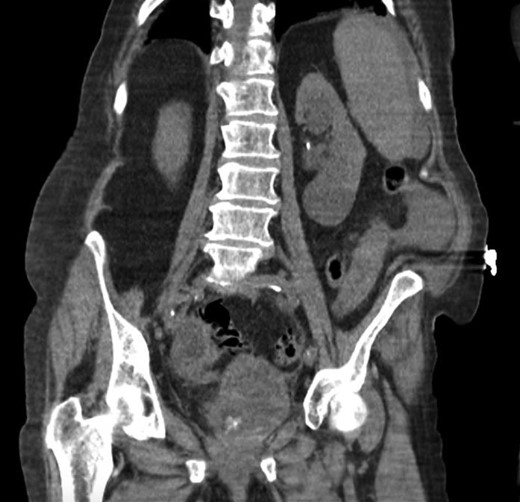
Coronal computed tomography showing a thickened inflamed bladder wall and irregular foci of hyperintensity within the bladder lumen.
Emergent exploratory laparotomy was performed. At surgery, the patient had gross succus peritonitis and a perforated pre-pyloric ulcer, which we repaired with a Graham patch. Upon further exploration of the patient’s abdomen, we found a large friable mass on the dome of the bladder, which was adhered to the rectum posteriorly. Friable tissue broke off from the dome with abdominal irrigation, making the Foley catheter balloon visible, and revealing tumor encompassing the entire bladder mucosa. A portion of the tumor was excised for biopsy, and the bladder was repaired with primary two-layer closure using 0-chromic suture and evaluated for leak with methylene blue via the Foley. A feeding jejunostomy tube and a pelvic Jackson-Pratt drain were placed.
The pre-pyloric ulcer pathological specimen showed acute and chronic duodenitis with fibrinopurulent exudate, ulceration, and prominent glands with lymphoid aggregates and reactive epithelial changes. The bladder mass showed an infiltrating moderately differentiated squamous cell carcinoma with large areas of necrosis invading into the fatty tissue. The bladder tumor was positive for the biological markers in Table 1, as well as for high risk HPV (16, 18, and 31).
| GATA-3 | L50-823 | GATA binding protein 3; nuclear expression in mammary and urothelial carcinomas | Negative |
| CK 5/6 | D5/16 B4 | Squamous and mesothelial cells | Positive |
| CK 903 | 34BE12 | CK 57, high moldecular weight 66kD | Positive |
| Thrombomodulin | 1009 | Mesothelioma, some epithelial tumors | Focally Positive |
| CDX2 | EPR27G4Y | Carcinoma subset, intestinal-type tumors | Negative |
| CEA monoclonal | TF3H8-1 | Carcinoembryonic antigen, adenocarcinoma | Negative, High Background |
| Chromogranin A | LK2410 | Neuroendocrine marker | Negative |
| PAX-8 | MRQ-50 | Ovarian serous carcinoma, thyroid and renal carcinoma, seminoma | Negative |
| CA125 | OC125 | Ovarian, breast, others | Negative |
| ER | SP-1 | Estrogen receptor | Negative |
| p16-INK4a | E6H4 | Cyclin-dependent kinase inhibitor, CIN/high grade SIL | Negative |
| GATA-3 | L50-823 | GATA binding protein 3; nuclear expression in mammary and urothelial carcinomas | Negative |
| CK 5/6 | D5/16 B4 | Squamous and mesothelial cells | Positive |
| CK 903 | 34BE12 | CK 57, high moldecular weight 66kD | Positive |
| Thrombomodulin | 1009 | Mesothelioma, some epithelial tumors | Focally Positive |
| CDX2 | EPR27G4Y | Carcinoma subset, intestinal-type tumors | Negative |
| CEA monoclonal | TF3H8-1 | Carcinoembryonic antigen, adenocarcinoma | Negative, High Background |
| Chromogranin A | LK2410 | Neuroendocrine marker | Negative |
| PAX-8 | MRQ-50 | Ovarian serous carcinoma, thyroid and renal carcinoma, seminoma | Negative |
| CA125 | OC125 | Ovarian, breast, others | Negative |
| ER | SP-1 | Estrogen receptor | Negative |
| p16-INK4a | E6H4 | Cyclin-dependent kinase inhibitor, CIN/high grade SIL | Negative |
| GATA-3 | L50-823 | GATA binding protein 3; nuclear expression in mammary and urothelial carcinomas | Negative |
| CK 5/6 | D5/16 B4 | Squamous and mesothelial cells | Positive |
| CK 903 | 34BE12 | CK 57, high moldecular weight 66kD | Positive |
| Thrombomodulin | 1009 | Mesothelioma, some epithelial tumors | Focally Positive |
| CDX2 | EPR27G4Y | Carcinoma subset, intestinal-type tumors | Negative |
| CEA monoclonal | TF3H8-1 | Carcinoembryonic antigen, adenocarcinoma | Negative, High Background |
| Chromogranin A | LK2410 | Neuroendocrine marker | Negative |
| PAX-8 | MRQ-50 | Ovarian serous carcinoma, thyroid and renal carcinoma, seminoma | Negative |
| CA125 | OC125 | Ovarian, breast, others | Negative |
| ER | SP-1 | Estrogen receptor | Negative |
| p16-INK4a | E6H4 | Cyclin-dependent kinase inhibitor, CIN/high grade SIL | Negative |
| GATA-3 | L50-823 | GATA binding protein 3; nuclear expression in mammary and urothelial carcinomas | Negative |
| CK 5/6 | D5/16 B4 | Squamous and mesothelial cells | Positive |
| CK 903 | 34BE12 | CK 57, high moldecular weight 66kD | Positive |
| Thrombomodulin | 1009 | Mesothelioma, some epithelial tumors | Focally Positive |
| CDX2 | EPR27G4Y | Carcinoma subset, intestinal-type tumors | Negative |
| CEA monoclonal | TF3H8-1 | Carcinoembryonic antigen, adenocarcinoma | Negative, High Background |
| Chromogranin A | LK2410 | Neuroendocrine marker | Negative |
| PAX-8 | MRQ-50 | Ovarian serous carcinoma, thyroid and renal carcinoma, seminoma | Negative |
| CA125 | OC125 | Ovarian, breast, others | Negative |
| ER | SP-1 | Estrogen receptor | Negative |
| p16-INK4a | E6H4 | Cyclin-dependent kinase inhibitor, CIN/high grade SIL | Negative |
The patient was evaluated by oncology, but was too ill for inpatient treatment, and was scheduled for outpatient follow-up. She was also evaluated by the palliative care team for pain management and goals of care discussion, which she declined. She was discharged to a rehabilitation facility on post-operative Day 18, but returned to the emergency department 9 days later in respiratory distress. She had an advanced directive with do not resuscitate/do not intubate (DNR/DNI) orders. Comfort measures were provided and the patient was pronounced dead after pulseless electrical activity on post-operative Day 27.
DISCUSSION
Urothelial carcinoma is the most common subtype of bladder cancer worldwide, accounting for 90–95% of all bladder carcinomas [1, 2]. It is one of the most common cancers in men, with the highest incidence in developing countries [1, 2]. Muscle invasion is one of the most important prognostic factors [1]. Risk factors include tobacco smoke, bladder irritants, benign prostate hypertrophy, urinary tract infections (UTI) (especially caused by Escherichia coli, Proteus mirabilis, and Streptococcus faecalis), and chronic inflammatory conditions [3]. Symptoms include hematuria, and urinary urgency, frequency, and obstruction [3].
Non-urothelial bladder carcinoma accounts for only 5–10% of bladder cancers [2]. It typically presents at a more advanced stage with generally worse overall survival than urothelial carcinoma [4]. Non-urothelial carcinoma originates from both epithelial (squamous cell carcinoma (SCC), adenocarcinoma, and small cell carcinoma) and non-epithelial (sarcoma, spindle cell carcinoma, and signet ring) cell types [2]. In general, the unique biology of non-urothelial bladder carcinoma is less understood [5].
SCC is an epithelial neoplasm defined by characteristic histological features such as squamous pearls, intercellular bridges, and keratohyalin granules [3]. The diagnosis is reserved for tumors without any evidence of urothelial components [3]. HPV has been linked to SCC, but this remains controversial [6, 7]. SCC accounts for 2–5% of all bladder cancers and has two common histological subtypes, bilharzial and non-bilharzial.
Bilharzial SCC is most commonly found in the Middle East, Southeast Asia, and South America. It usually presents in the fifth decade of life, predominantly in men, with hematuria. It is typically discovered at an advanced stage, with 50% low-grade and 18% lymph node metastasis. The standard surgical treatment is radical cystectomy and the overall 5-year survival rate is approximately 50–60%. Recurrence is mostly local, and is prevented with snail control and anti-bilharzial drugs [3].
Non-bilharzial SCC is most commonly found in western countries. It usually presents in the seventh decade of life, has a 3:2 male:female predominance, with hematuria. Risk factors include chronic indwelling urinary catheters and other bladder irritants, chronic inflammation, and chronic UTI. Non-bilharzial SCC is typically discovered at an advanced stage, mostly high-grade, and 8–10% with lymph node metastasis. Recurrence is mostly local and may be prevented by avoidance of bladder irritants [3]. The standard surgical treatment is radical cystectomy with an ileal conduit, and the overall 5-year survival is 33–48%. Radical cystectomy in men involves removal of the bladder, prostate, seminal vesicles, and proximal urethra, and in women involves removal of the bladder, urethra, uterus, fallopian tubes, ovaries, anterior vaginal wall, and surrounding fascia [8].
The presence of specific biomarkers and the lack of clinical evidence to suggest another primary origin such as anus, rectum, cervix, or uterus, support our conclusion that our patient had a very rare form of primary non-urothelial squamous cell carcinoma of the bladder [9, 10]. Her case provides a noteworthy example of a rare incidental finding during an emergent clinical presentation.
ACKNOWLEDGMENTS
None of the authors listed for this publication have any financial support or conflicts of interest.
CONFLICT OF INTEREST STATEMENT
None declared.



