-
PDF
- Split View
-
Views
-
Cite
Cite
Peng Zhang, Mojin Wang, Lifen Bai, Wen Zhuang, A unique case of ectopic pancreas presenting as jejunal malignance, Journal of Surgical Case Reports, Volume 2019, Issue 7, July 2019, rjz217, https://doi.org/10.1093/jscr/rjz217
Close - Share Icon Share
Abstract
Ectopic pancreas is defined as pancreatic tissues having no anatomic or vascular connections with the orthotopic pancreas. It is difficult for clinicians to diagnose this disease without performing a histopathological examination because it lacks specific clinical manifestations. This case report is of a 46-year-old woman who presented with epigastric pain. She had elevated serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 72-4 (CA72-4). Abdominal contrast-enhanced computed tomography (CT) revealed a persistently enhanced mass in the proximal jejunum, which was confirmed as ectopic pancreas via histopathological examination. Her serum CEA and CA72-4 levels were restored to normal ranges after resecting the jejunal ectopic pancreas. This is the first reported case of ectopic pancreas causing an elevation in serum CEA and CA-724 levels; this report supports the metaplasia theory and suggests that jejunal masses should be cautiously diagnosed for avoiding unnecessary concerns among patients and their families.
INTRODUCTION
Ectopic pancreas, also called heterotopic pancreas, is a type of congenital anomaly defined as pancreatic tissues lacking anatomic or vascular connection with the orthotopic pancreas. The prevalence of ectopic pancreas has been estimated to be 0.5–13.7%. It could occur either within the digestive tract or outside it in organs such as the liver, spleen, umbilicus, lung, and mediastinum. While it commonly occurs in the stomach (30–50%), it is comparatively less prevalent in the jejunum, with a reported frequency of only 13% [1]. Multiple complications occur secondary to ectopic pancreas including pancreatitis, pseudocyst formation, malignant degeneration, gastrointestinal bleeding, bowel obstruction, and intussusception [2]. Most patients with ectopic pancreas are asymptomatic, whereas a few patients complain of nonspecific symptoms, such as epigastric pain, nausea, and vomiting, which are suggested to be associated with its size, location, and accompanying complications [3]. Additionally, it lacks specific laboratory tests and imaging features. Here, we report a unique case of ectopic pancreas which is easily misdiagnosed.
CASE REPORT
A 46-year-old woman complaining of epigastric pain for 1 month was admitted to our hospital. She denied a history of abdominal diseases or surgeries. Her physical examination results were normal. Laboratory tests, including blood routine tests as well as tests for liver and kidney function, electrolytes, pertinent serum tumor markers, were within the normal ranges except for the levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 72-4 (CA72-4), which were elevated (Fig. 1). Therefore, further tests were performed. Esophagogastroduodenoscopy did not reveal anything abnormal; however, abdominal contrast-enhanced computed tomography (CT) demonstrated a persistently enhanced mass (12 mm × 8 mm in size) in the proximal jejunum (Fig. 2A). Therefore, the patient underwent an exploratory laparotomy owing to a suspicion of malignancy; a solid mass was located approximately 30 cm away from the Treitz ligament and protruded from the serosal surface. Intraoperative exploration revealed no other abnormality. Subsequently, segmental resection of the affected jejunum was performed. Surprisingly, intraoperative frozen section histopathological analysis of the resected specimen revealed pancreatic tissues; postoperative pathological examination of these pancreatic tissues confirmed the presence of jejunal ectopic pancreas containing numerous acini cells in the submucosa and muscular layer; however, malignant transformation or inflammation were not noted (Fig. 2B 200×, H&E). Thus, side-to-side anastomosis rather than extended resection was performed. More surprisingly, serum levels for the two tumor markers (CEA and CA72-4) decreased to normal levels when rechecked on the fifth day postoperatively (Fig. 1). The patient was discharged on the sixth day postoperatively, and no abnormality was noted during 6 months of follow-up.
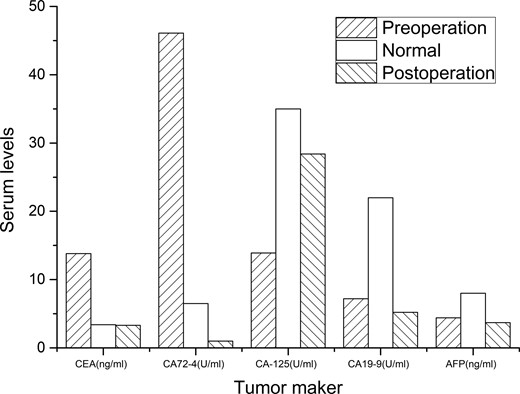
Changes in serum tumor maker levels. Carcinoembryonic antigen (CEA) and carbohydrate antigen 72-4 (CA72-4) levels were elevated preoperatively but were restored to the normal levels postoperatively. Carbohydrate antigen 125 (CA-125), carbohydrate antigen 19-9 (CA19-9), and alpha-fetoprotein (AFP) were normal both before and after surgery.
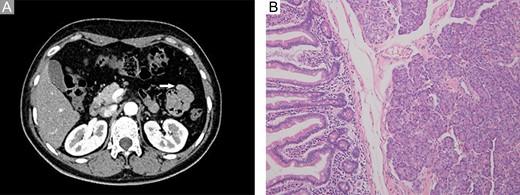
Imaging and pathological examination findings. (A) Computed tomography scan revealed a persistently enhanced mass (approximately 12 mm × 8 mm in size) located at the proximal jejunum (white arrow). (B) Histological diagnosis revealed ectopic pancreas dominated by acini within the submucosa and muscular layer; however, no malignant transformation or other complications were observed (200×, H&E).
DISCUSSION
The ectopic pancreas was first reported by Shultz in 1727. More than 100 years later, the histopathologic features of ectopic pancreas was described by Klob in 1859 [4]; however, its exact pathogenesis still remains controversial. There are two prevailing theories. One is the migration theory which suggests that fragments of pancreatic tissues separate from the developing pancreas and are deposited in anomalous regions during embryogenesis [5]. The other is the metaplasia theory which proposes that totipotent endodermal cells give rise to pancreatic tissues owing to the presence of some molecular abnormalities in aberrant locations [6]. In the past report, the serum tumor marker level associated with ectopic pancreas usually remains normal except in individuals who have a complication of malignant degeneration. However, the ectopic pancreas in this case caused an increase in serum CEA and CA-724 levels. To the best of our knowledge, a similar case has not been previously reported. According to the literature, an abnormal increase in serum CEA and CA72-4 levels implies the existence of gastrointestinal cancers, especially gastric cancer [7]. While some nonmalignant factors reportedly increase the circulating concentration of CEA and CA72-4, to our knowledge, the ectopic pancreas has never before been reported to do so [8]. The exact reason for this phenomenon was unclear. However, increased secretion of CEA and CA72-4 in serum indicates that some molecular abnormalities are present in the ectopic pancreatic tissue which may support the metaplasia theory.
Jejunal masses are usually diagnosed via imaging findings rather than digestive endoscopy before surgery owing to their peculiar location. The imaging features of ectopic pancreas correlate with its histologic composition. Lesions dominated by acini exhibit an enhancement greater than or similar to that of the orthotopic gland, whereas lesions dominated by ducts and hypertrophied muscle are less enhanced [2]. The jejunal mass in this case was dominated by acini and manifested as a persistently enhanced structure on an abdominal contrast-enhanced CT scan, which was similar to the findings for certain malignancies. The changes in serum CEA and CA-724 levels further increased the suspicion of malignancy. However, ectopic pancreas was the definitive diagnosis. Thus, the preoperative diagnosis of a jejunal mass should be made cautiously and intraoperative frozen section histopathological analysis of the resected specimen should be performed in a timely manner in order to avoid unnecessary extended resections and worries among patients and their families.
CONFLICT OF INTEREST STATEMENT
None declared.



