-
PDF
- Split View
-
Views
-
Cite
Cite
Masayasu Takegawa, Natsuko Kakudo, Naoki Morimoto, Masakatsu Hihara, Hiromu Masuoka, Kenji Kusumoto, Primary cutaneous adenoid cystic carcinoma on the lower leg, Journal of Surgical Case Reports, Volume 2019, Issue 6, June 2019, rjz201, https://doi.org/10.1093/jscr/rjz201
Close - Share Icon Share
Abstract
Primary cutaneous adenoid cystic carcinoma (PCACC) is a very rare malignant tumor. Here a case of PCACC on the left lower leg with metastasis to the inguinal lymph node. The tumor resection and the inguinal lymph node dissection were performed under general anesthesia, and the defect was covered with free meshed skin graft. No complication or recurrence has occurred after the surgery.
INTRODUCTION
Primary cutaneous adenoid cystic carcinoma (PCACC) is a rare malignant skin apppendageal tumor. It develops mostly on the head and neck. In addition, it is reported that the incidence of distant metastasis from the initial PCACC lesion is very low [1].
Here, an 83-year-old male case of PCACC on the lower leg with metastasis to inguinal lymph node is reported. The atypical fashions of PCACC, the initial location and the metastasis, are referred in this report.
CASE REPORT
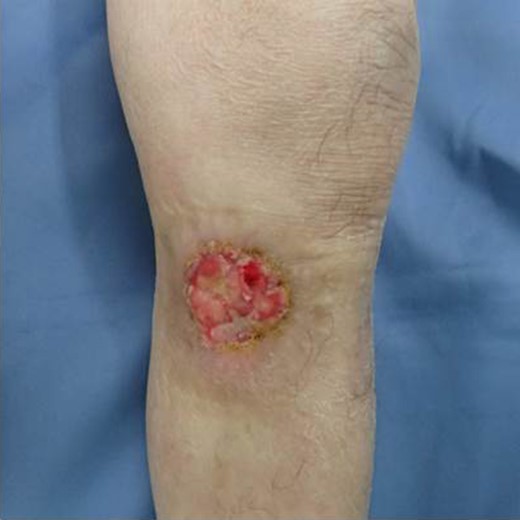
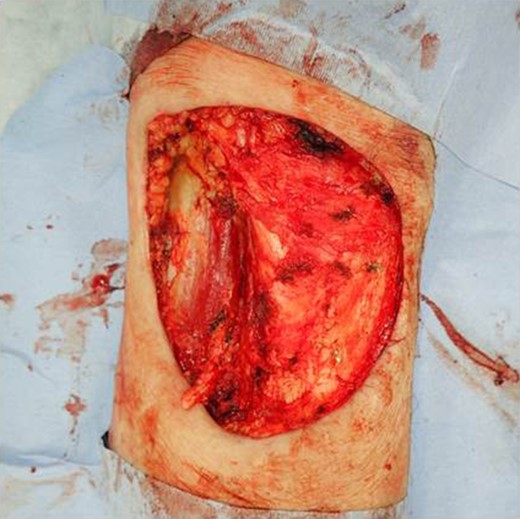
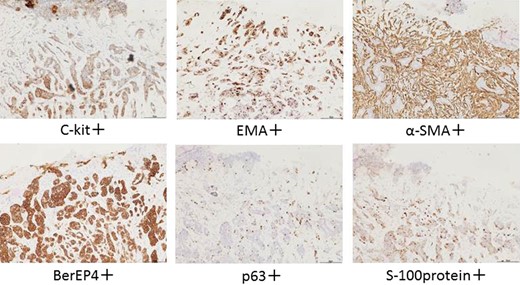
The patient was an 83-year-old male with a granulomatous tumor on his left lower leg. He said that it had existed for about 10 years. An incisional skin biopsy was performed at a neighbor medical institution and histopathologically suggested PCACC. He was introduced to our out-patient clinic. At his admission, the tumor was about 4 cm in the diameter and showed a few red granulomatous nodules (Fig. 1). MRI revealed that the tumor infiltrated subcutaneous tissue but did not reach muscle and bone (Fig. 2). The whole-body PET-CT showed that fluorodeoxyglucose (FDG) accumulated at the tumor area of the left lower leg. FDG also accumulated in the left inguen, however any other accumulation was not defected (Fig. 3). Therefore, it was suspected that the left inguinal hot spot was the metastatic lesion from the PCACC lesion at the left lower leg. At the first operation, the tumor was resected 2 cm far from the tumor margin, including basal muscle fascia and periosteum, and the defect was covered with an artificial dermis under general anesthesia (Fig. 4). At the same time, inguinal lymph node dissection was performed. Histopathological examination of the tumor revealed that cubical cells which had high nuclear-to-cytoplasmic ratio proliferated with cribriform, tubular or cord-like structure in the dermis and subcutaneous tissue. Perineural invasion by the tumor was observed. Metastasis to superficial inguinal lymph node was also histological confirmed (Figs 5 and 6). Most lumens had mucus which was stained by Alcian-blue (AL-B) and Periodic acid-Schiff (PAS) inside of them (Fig. 7). In the immunohistochemical findings, the tumor cells were positive for EMA, SMA, S-100protein, BerEP4, p63 and C-kit (Fig. 8). According to these clinical and pathological findings, the tumor was finally diagnosed the tumor as PCACC. Microscopically, the tumor was resected completely.
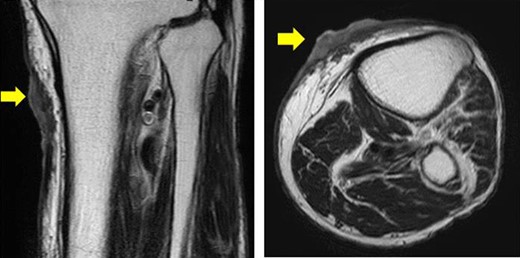
Horizontal and sagittal views on MRI T2 (yellow arrow indicates the tumor).
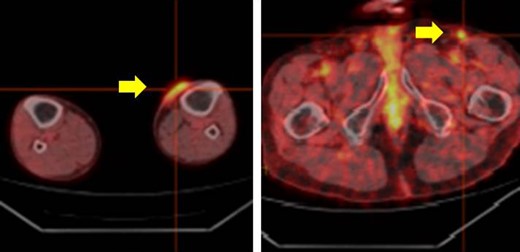
The whole body PET-CT (yellow arrow indicates accumulation of FDG).
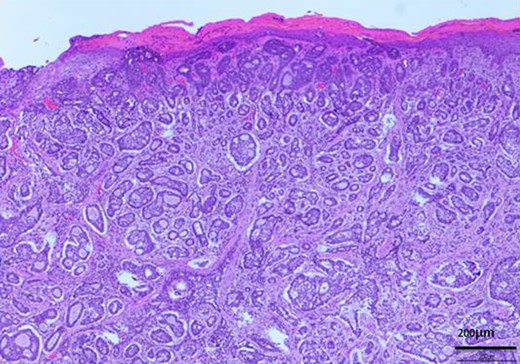
H-E stain revealed that cubical cells which had high nuclear-to-cytoplasmic ratio proliferated with cribriform, tubular or cord-like structure in the dermis and subcutaneous tissue.
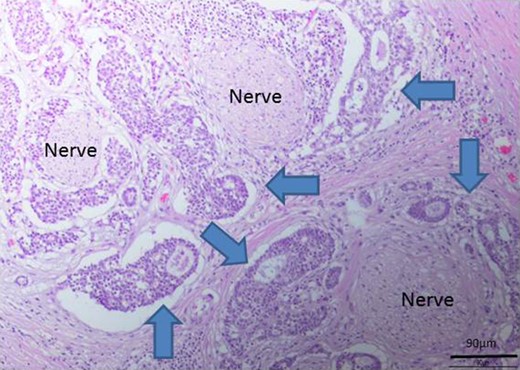
Perineural invasion by the tumor (blue arrows indicate the tumor).
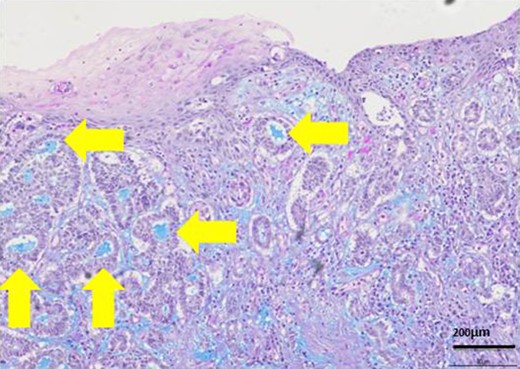
Most lumens had mucus which was stained blue by AL-B and PAS stain (yellow arrows). It showed lumens had acid mucopolysaccharide like sialomucin.
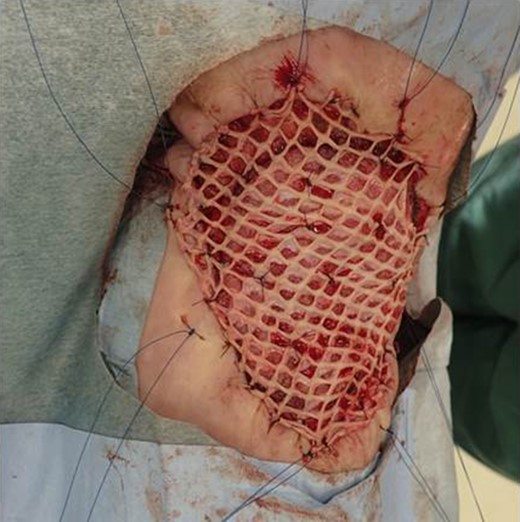
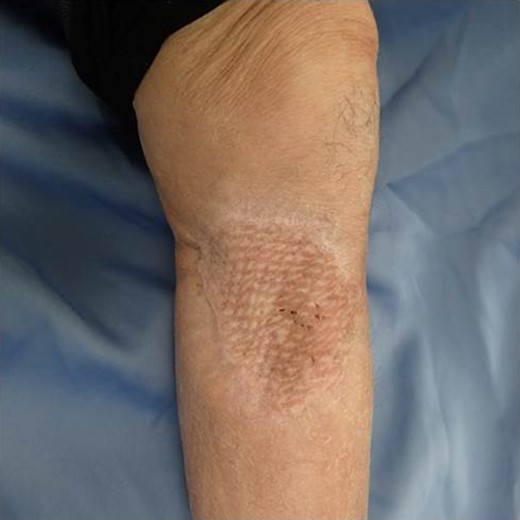
After one week from the first operation, the artificial dermis was removed because of much exudate and contamination of the wound. Since then, we washed it with saline every day. After one month from the first operation, it became clear, however, little granulomatous. So, we started negative pressure wound therapy to grow up the granulomas for 3 weeks. After two months from the first operation, wound bed preparation accomplished, and we performed additional resection with 1-cm resection margin and covered the defect by mesh skin graft which was taken from right inguen and thinned to 25/1000 inch (Fig. 9). The skin completely engrafted and no recurrence of the tumor was noted as of 1 year postoperatively (Fig. 10).
DISCUSSION
Primary cutaneous adenoid cystic carcinoma (PCACC) was first described as and of skin appendageal tumors by Boggio in 1975 [2]. The incidence of PCACC was reported as 0.23 per 1 million person-years in the US Surveillance, Epidemiology, and End Results (SEER) program [3]. PCACC occurs mainly on the head and neck, but on any part of body infrequently. Remarkrishnan et al. reported that PCACCs occurred [46% on the head and neck], [17% on the upper limbs], [15% on the trunk], [13% on the lower limbs]. They also reported that the frequency of distant metastases from the original PCACC lesion was very low, about 4% to the lymph node, and about 7% to the distant organ [1]. Therefore, it can be said that our case was very rare one out of PCACCs.
Seab et al. stated that the diagnosis of PCACC needed the points; (1) presence in the dermis, (2) cribriform pattern which consisted of basaloid cells, (3) fibromucinous stroma, (4) lumen which had sialomucin, (5) not originated from salivary glands [4]. Our case fulfilled these points, and then was typical in PCACC.
Immunohistochemically, Alkan BI et al. reported in his case study that PCACC showed BerEp4, CEA, CD117(C-kit), and CK7 expression in the regions neighboring the luminal areas, and p63 and SMA positivity were detected in myoepithelial cells at the periphery of the cell islands. He said the two different cell populations provided an important clue for diagnosis because the tumor cells showed ductal and myoepithelial differentiation [5]. In fact, these features helped us for diagnosis in this case.
The standard treatment of PCACC is a wide surgical excision with at least a 2 cm safety margin from the tumor, margin to avoid its frequent recurrence [6]. In this case, tumor was completely resected more than 2 cm far from the tumor margin, and no recurrence of the tumor was noted as of 1 year postoperatively. We should discuss the margin of the PCACC warily at the operation time, and two-staged reconstruction is recommended, especially in a large or long elapsed tumor.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
None declared.
FINANCIAL DISCLOSURES
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.



