-
PDF
- Split View
-
Views
-
Cite
Cite
Saeed Bahabri, Ammar C Al Rikabi, Amjad O Alshammari, Sara I Alturkestany, Hemophagocytic lymphohistiocytosis associated with Epstein–Barr virus infection: case report and literature review, Journal of Surgical Case Reports, Volume 2019, Issue 6, June 2019, rjy096, https://doi.org/10.1093/jscr/rjy096
Close - Share Icon Share
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is rare and life threatening syndrome. There are only a few reported cases of HLH with GI symptoms. We describe the case of an 18 months old boy who presented with a history of fever for 40 days, abdominal distention and hepatosplenomegaly. Abdominal x-ray showed a pneumoperitoneum. Urgent laparotomy was done which revealed an isolated cecal perforation. The histopathological findings in the subsequent resected bowel was HLH with evidence of positive EBV Barr infection.
INTRODUCTION
Hemophagocytic lymphohistiocytosis (HLH) is considered to be an atypical but possibly deadly syndrome defined by uncontrolled and inefficient activation of the mononuclear phagocytic system resulting in an uncontrolled hyper-inflammatory response. HLH can be either primary or secondary. Primary HLH, also known as familial HLH, frequently appears in childhood and it is generally caused by genetic defects in proteins involved in the cytotoxic function of T-lymphocytes and natural killer (NK) cells. Secondary HLH, also called sporadic HLH, arises in both children and adults and is commonly related to an underlying infectious, autoimmune or malignant etiology [1]. Patients with HLH present with an ample spectrum of clinical manifestations and their conditions may speedily deteriorate, leading to considerable morbidity and mortality [2]. Treatment emphasizes on controlling the underlying cause and administrating immunosuppressive, immunomodulatory and cytotoscie medications. The disease has a high mortality if left untreated, and for this reason, immediate diagnosis and initiation of treatment are essential to boost the chances of survival in affected patients [3].
CASE REPORT
An 18 months old boy who presented with a history of fever for 40 days duration was referred to our hospital. Physical examination showed abdominal distention and subsequence x-rays revealed a pneumoperitoneum. Abdominal U/S also showed hepatosplenomegaly. The patient underwent urgent laparotomy and was found to have an isolated cecal perforation. Resection and anastomosis were done. Past medical history showed several visits to health centers where the patient was evaluated and received many courses of antibiotics without any improvement. His laboratory investigations showed a drop of WBCs and a negative Epstein–Barr virus (EBV) serology result.
GROSS AND HISTOPATHOLOGICAL FINDINGS
The excised cecal perforation consisted of an unoriented segment of bowel measuring 2 cm in maximum diameter with a small serosal perforation site. Histopathological sections showed a piece of colonic mucosa and wall with extensive transmural mononuclear inflammatory cells infiltrate. The cells are arranged in nodules consisting of histiocytes and lymphocytes (Fig. 1A and 1B). A panel of immunohistochemical stains was done which revealed that the cells within the nodules are predominantly positive for CD68, Fig. 2A also positive for both B and T lymphocytes with predominant B cell linage. The histiocytes within the nodule were found to be positive for EBV immunostain (Fig. 2B).
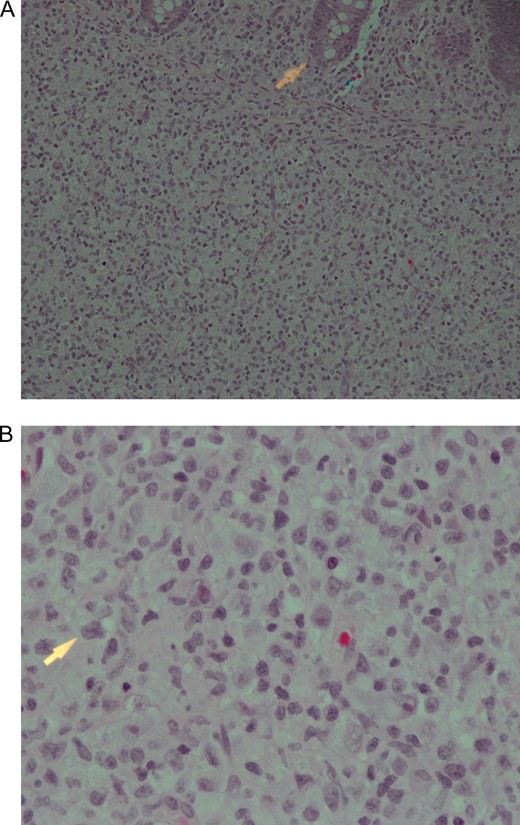
(A) Submucosa nodule consisting of mixed inflammatory cells including numerous histiocytes with many B and T lymphocytes. The arrowhead is pointing towards the edge of a mucosal crypt. Haematoxylin and eosin stain ×200. (B) High power view of the nodule described in (A). Note the presence of some pleomorphic cells showing prominent nucleoli. Haematoxylin and eosin stain ×600.
DISCUSSION
HLH is a serious condition of acute hyper inflammation occuring as a result of uncontrolled proliferation of activated lymphocytes and histiocytes secreting high amounts of inflammatory cytokines. A pathophysiological disturbance causes a hyper-inflammatory response due to cytokine deregulation with impaired T cells and NK cells function and hemophagocytosis due to activation of macrophages [4]. A case similar to our case reported by S.popeskou. It showed a 26 years old male patient who is a known case of Crohn’s disease. He was admitted to the hospital complaining of fever and sore throat due to infectious mononucleosis associated with EBV viremia and was also found to have severe lower GIT bleeding. Colonoscopy revealed severe pancolitis. Biopsies from the stomach, duodenum and colonic mucosa showed activated histiocytes which are positive for EBV infection and consistent with HLH [4]. Another case of HLH was described in a 42 years old women who developed HLH shortly after influenza virus (H1N1) infection. The skin biopsy taken from this patient showed perivascular lymphocyte and macrophages consistent with H1N1 induced Hemophagocytic lymphohistiocytosis HLH syndrome. Viral induced HLH include EBV, H1N1, CMV, herpes simplex, adenovirus and H5N1 [4, 5]. Genetic (primary) HLH is inherited as an autosomal recessive or X-linked types. It consists of two subgroups: familial LH (FHLH), which was first defined by Farquhar and Claireaux in 1952, and the distinct immune deficiencies including Chédiak-Higashi syndrome 1 (CHS-1) (OMIM 214500), Griscelli syndrome 2 (GS 2) (OMIM607624) and X-linked proliferative syndrome (XLP) (OMIM 308240) [6]. Adult-onset HLH is often secondary to an underlying disease, like infection, malignancy, or autoimmune disease [2]. Patients with primary HLH present with the following symptoms in order of frequency fever (91%), hepatomegaly (90–95%) and splenomegaly (84%) while others may present with neurological symptoms (47%), rash (43%) and lymphadenopathy (42%). Furthermore, neurological symptoms (33%) may show CSF abnormalities such as spinal fluid mononuclear infiltrationand/or elevated spinal fluid protein [7]. To the best of our knowledge, this case represents the first reported case of intestinal perforation secondary to HLH (Fig. 2).
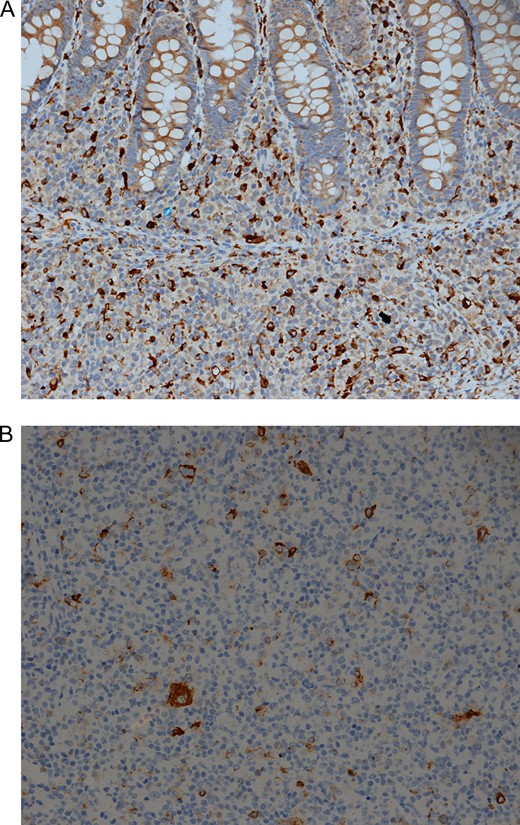
(A) Immunohistochemical stain for the histiocytic marker CD68 is positive in a large number of histiocytes IHC for CD68 ×200. (B) Immunohistochemical stain for Epstein Barr virus. Note the strong positive membranous / cytoplasmic staining in several cells. IHC EBV ×200.
CONCLUSION
In young children presenting with acute abdomen secondary to bowel obstructon and perforation, the possibility of HLH should be suspected and investigated. EBV infection as a causative factor should be confirmed by serology and immunohistochemical stains.
CONFLICT OF INTEREST STATEMENT
None declared.



