-
PDF
- Split View
-
Views
-
Cite
Cite
Khalid N Shehzad, Sherif Monib, Mussa Mensa, Mustafa O Halawa, Bilateral persistent sciatic arteries complicated by unilateral acute lower limb ischaemia, Journal of Surgical Case Reports, Volume 2019, Issue 4, April 2019, rjz119, https://doi.org/10.1093/jscr/rjz119
Close - Share Icon Share
Abstract
We present a case of a middle-aged Caucasian woman who developed acuteon top of chronic limb ischaemia secondary to thrombotic occlusion of a persistent sciatic artery (PSA). Timely investigation and treatment were instituted resulting in a favourable outcome. PSA is an uncommon congenital, developmental, arterial anomaly which can cause serious lower limb complications such as acute or critical limb ischaemia and amputation. As this condition is rarely encountered in regular clinical practice, and has a limb-threatening potential, it is important to be aware of its cause, presentation and management. We describe the embryologic aetiology of PSA and discuss different investigation modalities and treatment options.
INTRODUCTION
Persistent sciatic artery (PSA) is a rare congenital vascular anomaly with fewer than 200 reported cases since it was first described in 1832 [1].
The embryological basis of this unusual anatomical variant was first elucidated in 1919 [2].
The embryonic sciatic artery arises from the umbilical artery at around the 6 mm embryo stage to supply the developing lower limb bud. With continuing development, it passes along the dorsal aspect of the growing skeletal mesenchyme and runs all the way to the sole of the foot. Segments of the embryonic sciatic artery are destined to persist as the popliteal and peroneal arteries. By the 12 mm embryo stage, the external iliac artery has arisen from the lateral aspect of the umbilical artery, just proximal to the origin of the sciatic artery, and started developing into the common and superficial femoral arteries, the latter terminating above the knee. The site of origin of the external iliac artery marks the subdivision of the umbilical artery into two parts—proximally, the future adult common iliac artery, distally the internal iliac artery. The sciatic artery develops a convexity towards the distal superficial femoral artery and fuses with the latter’s superior communicating branch just above the knee. Thereafter, the sciatic artery starts involuting so that by the 18 mm embryo stage, blood flows into the popliteal artery predominantly through the superficial femoral artery (SFA) and its superior communicating branch. By the 22 mm stage, the sciatic artery has completely obliterated, persisting only proximally as the inferior gluteal artery (Fig. 1) [2–4].
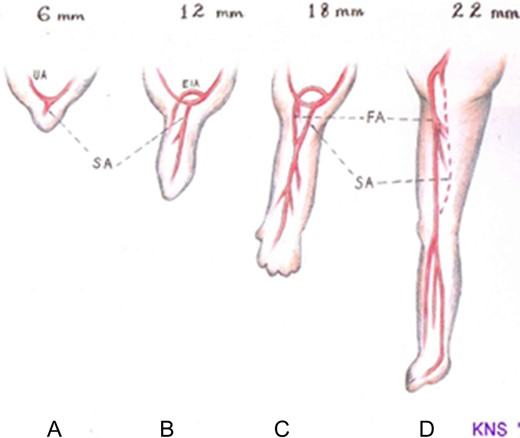
Schematic drawing showing the normal embryonic arterial development of the lower limb. (a) At the 6 mm embryo stage, the primitive sciatic artery (SA) has arisen from the umbilical artery (UA) and begins to supply the limb bud. (b) At the 12 mm embryo stage, the external iliac artery (EIA) develops from the umbilical artery proximal to the origin of the sciatic artery. (c) At the 18 mm embryo stage, the EIA extends into the common femoral artery (FA) which then continues as the superficial femoral artery after giving off its profunda branch. The sciatic artery develops a communication with the distal superficial femoral artery and itself begins to involute. (d) At the 22 m embryo stage, the sciatic artery has completely involuted save for its proximal end which persists as the inferior gluteal artery. The femoral artery is now the dominant blood supply to the lower limb.
It has been postulated that the sciatic artery may persist if there is incomplete formation of the femoral artery. However, reasons for the underdevelopment of the femoral artery remain unclear. Failure of the sciatic artery to regress can result in it becoming the dominant blood supply to the lower extremity in the form of a PSA [5].
Typically in cases of PSA, the internal iliac artery is tortuous and larger in diameter compared to the external iliac [3, 5].
The sciatic artery travels through the greater sciatic foramen below the piriformis muscle to enter the thigh, where it may accompany the posterior cutaneous nerve or lie within or adjacent to the sheath of the sciatic nerve, following a course along the posterior aspect of the adductor magnus muscle before reaching the popliteal fossa where it continues as the popliteal artery [5, 6].
PSA can broadly be classified into two types, complete and incomplete8. In complete PSA, the artery is the principal blood supply to the lower limb, and the SFA is generally hypoplastic, only providing collateral supply and terminates in the thigh. With incomplete PSA, the artery is underdeveloped and the SFA is the primary blood supply to the lower limb. PSA can be further classified into five different subtypeswith varying degrees of development and dominance between the SFA and PSA [5].
In a recent review by Van Hooft et al., incidence of PSA is estimated to be between 0.025-0.05% in angiographic studies [5].
PSA has a mean age of presentation of 54 years and does not appear to show any gender predilection [7, 8].
It is bilateral in approximately 30% of cases. PSA is associated with a number of complications including aneurysm formation, thromboembolism causing critical limb ischaemia and neuropathy secondary to sciatic nerve compression [5].
CASE
A 52-year-old Caucasian woman presented to the emergency department with a 3-day history of severe right leg pain associated with a cold and pale foot. Prior to this episode, she described a two-month history of mild intermittent claudication. There was no history of trauma. Pertinent past medical history included hypertension, a significant smoking history of approximately 34-pack-years and obesity. She had a family history of ischaemic heart disease, peripheral vascular disease and hypertension in first degree relatives. Physical examination revealed normal femoral pulses bilaterally as well as normal popliteal, dorsalis pedis and tibialis posterior pulses on the left. On the right, the popliteal, tibialis posterior and dorsalis pedis pulses were absent in association with a cold foot and a capillary refill time of approximately 5 seconds. There were no palpable masses in the lower extremities or gluteal region. There was diminished sensation in the right lower extremity in the distribution of the common and superficial peroneal nerve. There was no motor deficit.
In anticipation of arterial occlusive disease, an urgent CT angiogram of the aorta and lower extremities was performed with unexpected results. Bilateral sciatic arteries supplying the popliteal arteries were identified (Figs 2A and 3A). Native bilateral superficial femoral arteries were present but small in comparison. The right sciatic artery was occluded at the sciatic notch (Figs 2B and 3B) with reconstitution of the artery within the mid thigh. Distal to this, the posterior tibial and peroneal arteries were patent. The proximal anterior tibial artery was patent, but distally occluded. There was no stenosis or occlusion on the left, with three-vessel run off below the knee.
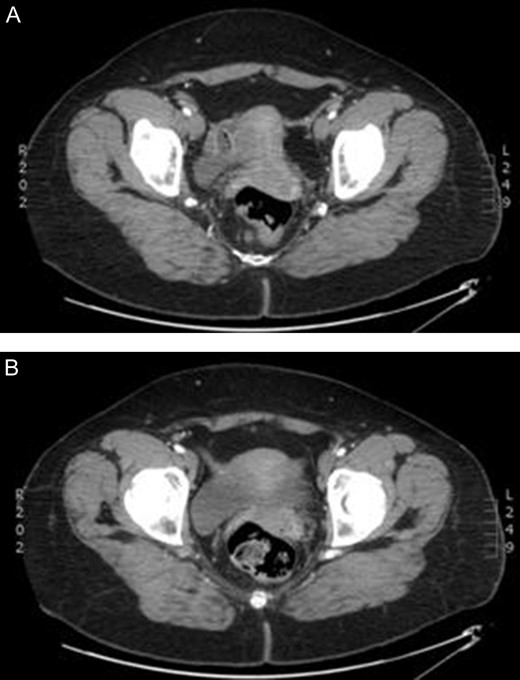
Enhanced computed tomography scan of a 52 year old woman with right lower limb ischaemia showing (A) bilateral persistent sciatic arteries entering greater sciatic foramen, and (B) a few slices inferiorly a thrombus occluding the right sciatic artery.
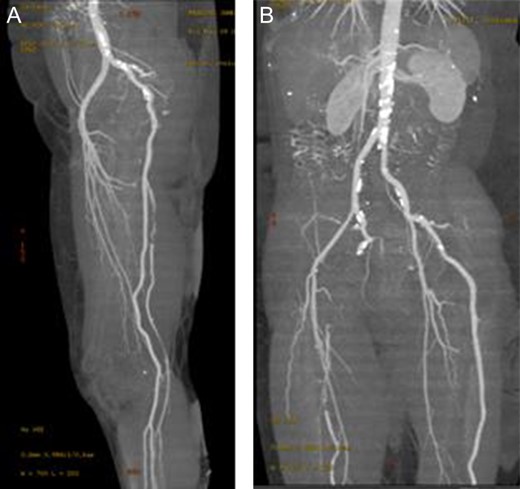
Enhanced three-dimensionally reconstructed computed tomography angiogram, showing (A) the internal iliac artery continuing as the sciatic artery and popliteal artery bilaterally, with hypoplastic superficial femoral arteries supplying the upper thigh. The angiogram also demonstrates (B) the occlusion in the right sciatic artery at the sciatic notch, with reconstitution in the distal thigh).
The patient was started on an infusion of low-molecular weight heparin whilst awaiting definitive treatment. The patient underwent a right sided femoro-popliteal bypass using a 8 mm polytetraflouroethylene (PTFE) prosthetic graft. The procedure was well tolerated and without complication. Post-operatively, an arterial duplex scan showed biphasic flow in the graft distally at knee level as well as in the native right tibialis posterior artery. Clinically, she had a good posterior tibial pulse on Doppler examination.
At follow-up appointments 6 weeks, 3 months and 9 months following the procedure, the patient remained well and reported no complaints or symptoms of claudication. The posterior tibial pulse remained detectable with Doppler, and graft patency was confirmed with Duplex ultrasound.
DISCUSSION
PSA is a rare developmental vascular anomaly that is often asymptomatic until a complication develops. The majority of cases of asymptomatic PSA are not discovered6 and those that are, are incidental findings. Aneurysm formation is the most frequent complication of PSA, occurring in up to 47% of cases [5, 8].
Typically, the aneurysm develops in the buttock between the piriformis muscle and the posterior aspect of the greater trochanter of the femur,[9] presenting as a pulsatile gluteal mass. The reason why these arteries are prone to aneurysm formation remains unclear. One possible hypothesis is that the artery is repeatedly overstretched and compressed during hip flexion as a consequence of its unusual posterior anatomical position [5].
Other studies have suggested that congenitally reduced elasticity in the arterial wall may play a role.
Aneurysms can lead to more severe complications including thrombosis, embolization and radicular pain, as well as amputation if not treated early [5]. A pulsatile buttock mass is pathognomonic for PSA [8].
Anatomically a PSA is in close proximity to the sciatic nerve, and a common complication is neuropathy secondary to nerve compression i.e. sciatica. This can make the clinical diagnosis of PSA difficult as reported symptoms are often indistinguishable from sciatica [8].
The incidence of stenosis and occlusion of PSA, leading to limb ischaemia, has been reported as 7% and 9%, respectively and an amputation rate of 8% [5].
Diagnosis of PSA relies on the patient’s presentation and a good physical examination. Patients present with symptoms of lower limb ischaemia (claudication/rest pain) due to thrombosis or distal embolisation from the PSA, a pulsatile buttock mass or sciatic neuropathy. Cowie’s sign (absence of femoral pulse and presence of distal pulses) may suggest a PSA but was absent in our patient as she had present, though small, femoral arteries bilaterally [10]. Definitive diagnosis is made by contrast angiography (CT/MR). Colour duplex scanning may not provide sufficient information [5].
PSA should be treated if it is causing symptoms. A PSA aneurysm can be obliterated by ligation, resection, embolisation or endovascular stenting [10].
Acute lower limb ischaemia caused by thrombotic occlusion of the PSA may be treated with arterial thrombolysis, but usually requiresa vascular reconstructive procedure such as femoro-popliteal bypass (in case of present femoral artery as in our case), iliopopliteal transobturator bypass or interposition graft [10].
Asymptomatic PSA patients should be monitored with interval physical examination and colour duplex scanning in order to detect aneurysm and/or ischaemia early [5, 10].
CONCLUSION
In conclusion, PSA is a rare congenital arterial anomaly with a high incidence of complications such as aneurysm and lower limb ischaemia. Its treatment depends on the patient’s symptomatology. Arterial bypass or—in case of PSA aneurysm—endovascular intervention may be employed to treat a symptomatic PSA. Asymptomatic PSAs should be regularly monitored to timely detect the development of complications.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest regarding the publication of this paper.
ETHICAL APPROVAL
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
AUTHOR’S CONTRIBUTIONS
All authors contributed in patient’s management, literature review as well as manuscript editing.
This article has not been presented at any symposium or conference to date.



