-
PDF
- Split View
-
Views
-
Cite
Cite
Nathan D Vandjelovic, Patrick C Barth, Stephen P Dunn, Kudakwashe R Chikwava, Udayan K Shah, Post-transplant lymphoproliferative disease of the larynx, Journal of Surgical Case Reports, Volume 2019, Issue 4, April 2019, rjz111, https://doi.org/10.1093/jscr/rjz111
Close - Share Icon Share
Abstract
Laryngeal post-transplant lymphoproliferative disease (PTLD) is rare. Here, we describe two pediatric cases. The first, a 15-month-old who underwent liver transplantation at 5 weeks, presented with airway distress. Airway evaluation identified epiglottic and arytenoid infiltrate, and biopsy was consistent with polymorphic PTLD. The second, a 23-month-old who underwent liver transplantation at 13 months, presented with progressive stridor. Airway evaluation revealed sub-mucosal infiltrate of the epiglottis, arytenoids, post-cricoid region, and uvula. Biopsy was consistent with monomorphic PTLD. Airway findings and symptoms resolved for both after immunosuppression reduction. PTLD diagnosis requires a high index of suspicion in post-transplant patients with airway obstruction.
INTRODUCTION
Post-transplant lymphoproliferative disorder (PTLD) is a rare, potentially fatal spectrum of abnormal lymphocyte or plasma cell proliferation occurring after solid organ or hematopoietic stem cell transplantation. The risk of developing PTLD after transplant is around 4.4% [1]. Before the diagnosis of PTLD, other lymphoplasmacytic infiltrations, such as those associated with infection, graft rejection, graft-versus-host disease, or lymphoma recurrence, must be ruled out [2]. Post-transplant immunosuppression is necessary to prevent organ or graft rejection. While immunosuppressed, immune cells are particularly susceptible to Epstein–Barr virus (EBV) infection, and uncontrolled proliferation of the affected immune cells can result. In EBV-negative PTLD, the etiology is unknown. Undetected EBV infection, other infections agents, chronic immune triggering by the graft, and genetic mutations have been suggested [2].
The World Health Organization classifies PTLDs into four histopathologically based categories [3]. The monomorphic and classical Hodgkin lymphoma type PTLDs are classified according to their corresponding leukemia or lymphoma counterparts as seen in immunocompetent hosts. Most non-destructive, polymorphic, and classical Hodgkin lymphoma-type PTLDs are EBV positive, whereas the percentage of EBV positivity is variable in monomorphic PTLD [4].
PTLD may occur during immunosuppression and is classically associated with cyclosporine and tacrolimus [5]. The median time to develop PTLD for all ages and comorbidities is 10 months [5]. Presentation is slightly shorter in children (8.1 months) and much longer in EBV-negative PTLDs (50 months) [5]. Initial symptoms range from painless lymphadenopathy to fevers, chills, night sweats, weight loss, and malaise. Primary manifestation of PTLD as upper airway obstruction has been reported as high as 75%, usually corresponding to hypertrophy of Waldeyer’s ring [6]. Laryngeal presentations are rare. To understand this unusual presentation of PTLD better, we present two cases of PTLD involving the larynx and review the literature on this topic.
CASE REPORT
Case 1
A 15-month-old male who underwent orthotopic liver transplant for idiopathic fulminant hepatic failure at age 5 weeks was maintained on tacrolimus without complication. He presented with a 6-month history of progressively worsening stridor. Airway fluoroscopy revealed mild tracheomalacia and irregular thickening of the epiglottis and aryepiglottic folds.
On direct laryngoscopy and bronchoscopy, circumferentially polypoid changes extending around the epiglottis and along the aryepiglottic folds were noted (Fig. 1). Biopsies of the epiglottis and right arytenoid revealed dense subepithelial lymphoplasmacytic infiltrates, composed of small and transformed lymphocytes, numerous plasma cells, admixed histocytes, and rare eosinophils. There was no cytologic atypia. Immunohistochemical stains revealed a mixture of T- and B-lymphocytes. Epstein in situ hybridization stains for Epstein-Barr virus mRNA (EBER) were negative. Observed histologic features (Fig. 2), together with response to immunosuppression reduction, were compatible with EBV-negative polymorphic PTLD.
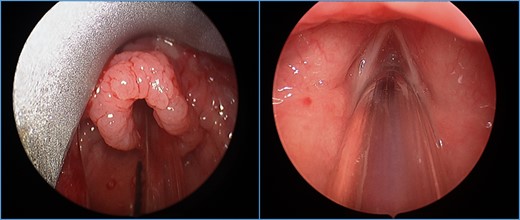
Polypoid changes noted circumferentially around the epiglottis extending to the aryepiglottic folds. Bilateral vocal folds visualized without changes.
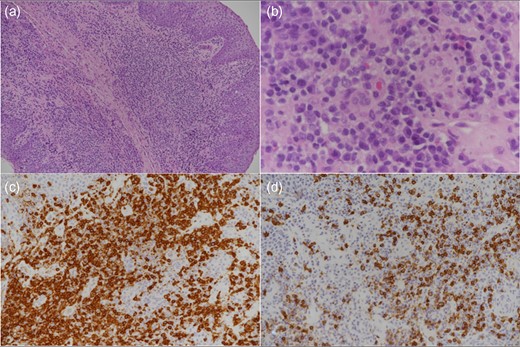
Low-power view (a) shows a dense subepithelial lymphoplasmacytic infiltrate and higher power view (b) shows admixed large transformed cells and rare eosinophils. CD3-positive T-cells (c) greatly outnumber CD20-positive B-cells (d).
Tacrolimus immunosuppression was discontinued and prednisolone was started. Ultimately, he was weaned from prednisolone and started on sirolimus. Within five days, the stridor resolved, and bedside flexible fiberoptic laryngoscopy showed improvement in the polypoid changes. Laryngoscopy seven months after the first procedure revealed normalization of the supraglottic tissue.
Case 2
A 13-month-old girl underwent liver transplant from her mother for biliary atresia. She required cadaveric liver retransplantation one week later due to poor allograft function. Soon after cadaveric transplantation, she developed moderate steroid-resistant acute rejection and was treated with thymoglobulin, tacrolimus, and mycophenolate. At 10 months post-transplantation (age 23 months), she presented with progressive stridor and dysphonia. She underwent liver biopsy, direct laryngoscopy with biopsy, and bronchoscopy, which revealed sub-mucosal infiltrates of the epiglottis, arytenoids, post-cricoid region, and uvula with normal vocal cord and tonsillar appearance (Fig. 3). Histopathology of the upper airway biopsies discovered variable subepithelial lymphoid proliferations. Immunohistochemical stains revealed the abnormal cells to be CD4-positive T-cells, including many follicular helper T-lymphocytes. EBER stains were negative. Flow cytometry and molecular studies showed no evidence of monoclonality. The histologic and immunohistochemical features (Fig. 4) were consistent with EBV-negative polymorphic PTLD. There was no evidence of PTLD or acute cellular rejection in the liver. Mycophenolate was discontinued, the tacrolimus dose was halved, and the intensity of the stridor improved.
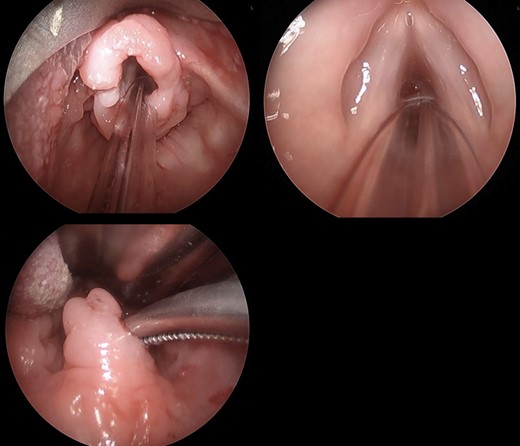
Infiltrate involving the supraglottic tissue but sparing the glottis. Irregular uvula with infiltrate.
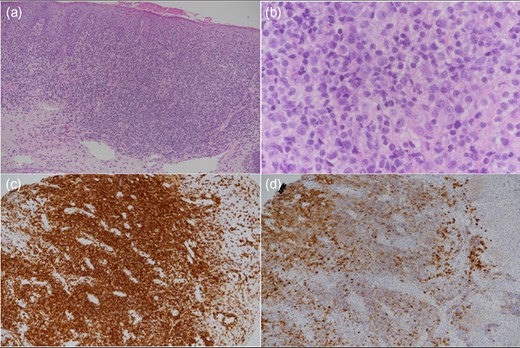
Low-power image (a) showing a dense subepithelial lymphoid infiltrate and higher power image (b) showing nuclear atypia in the small lymphoid cells and admixed larger transformed cells. CD3-positive T-cells (c) greatly outnumber CD20-positive B-cells (d).
After 1 month of reduction of immunosuppression, airway evaluation was repeated. Mild infiltration was appreciated on the epiglottis, arytenoids, and false vocal folds. Repeat biopsy of this tissue identified improved T-cell infiltrates; however, liver biopsy demonstrated moderate T-cell mediated rejection. The tacrolimus dose was increased and steroids were added. On the most recent follow-up, no stridor or increased work of breathing was recorded.
DISCUSSION
PTLD occurs due to unchecked proliferation of lymphoid and/or plasmacytic cells in the setting of immunosuppression. Hypertrophy of Waldeyer’s ring, which may lead to upper airway obstruction, has been reported in 83% of patients with PTLD [6]. Less commonly, laryngeal infiltration leads to upper airway symptoms, such as stridor and dyspnea. Redmann et al. reported that 13 of 158 patients with PTLD after either liver transplant or heart transplant presented with stridor, stertor, or sleep-disordered breathing, all had abnormal supraglottic findings [7]. Consistent with the presentation of both patients in this series, multiple other case studies associate the presentation of stridor or dyspnea with laryngeal involvement [6, 8–10].
Upper airway evaluation with biopsy is critical for an expedient diagnosis. Both children in this series underwent prompt operative laryngoscopy with biopsies. Airway visualization also contributes to the decision to secure the airway. Although neither of our patients required tracheostomy, the need, related to mass effect or difficulty with extubation, has been reported [6, 7]. We recommend follow-up laryngoscopy with biopsy to confirm disease resolution before further altering the immunosuppressive regiment.
Interestingly, EBV staining was negative in both cases. EBV-negative PTLD is less common than EBV-positive PTLD and is usually associated with monomorphic PTLD [5]. Monomorphic PTLD seems to be an adverse prognostic indicator, and although there may be an association between EBV class and outcome, this is difficult to delineate without a large cohort [5]. EBV-negative airway PTLD presentation and clinical progression have been described similarly to our cases. [6]
Reduction of immunotherapy alone will typically lead to remission [4]. Where immunotherapy reduction failed, rituximab alone has induced complete remission in one-third of cases; if necessary, CHOP (cyclophosphamide, hydroxy doxorubicin, vincristine, and prednisone) can be added to rituximab [2, 4]. Reduction of immunosuppression effectively reduced symptoms and histopathologic evidence of disease in our cases.
CONFLICT OF INTEREST STATEMENT
The authors declare that no conflicts exist to disclose.
FUNDING
No financial support was provided for this study.
REFERENCES
- airway obstruction
- biopsy
- arytenoid cartilage
- epiglottis
- liver transplantation
- lymphoproliferative disorders
- pediatrics
- therapeutic immunosuppression
- natural immunosuppression
- diagnosis
- larynx
- mucous membrane
- transplantation
- uvula of palate
- stridor
- posttransplant lymphoproliferative disorder
- airway device
- airway structure
- infiltrates



