-
PDF
- Split View
-
Views
-
Cite
Cite
Meredith Gunder, Vladimir Lakhter, Kwan Lau, Sunil S Karhadkar, Antonio Di Carlo, Riyaz Bashir, Endovascular intervention for iliac vein thrombosis after simultaneous kidney-pancreas transplant, Journal of Surgical Case Reports, Volume 2019, Issue 4, April 2019, rjz024, https://doi.org/10.1093/jscr/rjz024
Close - Share Icon Share
Abstract
May–Thurner syndrome (MTS) is an anatomic variant where the overlying right common iliac artery compresses and chronically obstructs the left common iliac vein, leading to thrombosis. Interventions for symptomatic MTS include endovascular thrombectomy and stenting. Occluding venous thrombus can be fatal to transplanted allografts. No guidelines exist for patients with MTS after simultaneous kidney-pancreas transplant. A 57-year-old female with ESRD and diabetes mellitus underwent a kidney-pancreas transplant. Post-operative imaging revealed a compressed left CIV with an occlusive thrombus threatening the renal graft. Thrombectomy with stent placement was performed, maintaining patency of both allograft venous outflows. Post-intervention the patient has demonstrated preserved kidney and pancreas allograft function through 1 year of follow-up. Interventions for MTS in patients after transplant are challenging given the complex allograft vascular reconstruction. We present a case which demonstrates that angiographic interventions for MTS can be safely performed after simultaneous kidney-pancreas transplant.
INTRODUCTION
Left common iliac vein (CIV) compression by the overlying right common iliac artery (CIA), or May–Thurner syndrome (MTS), is an anatomic variant described in over 20% of the population [1]. While the majority of this patient population is asymptomatic, this compression can lead to clinically significant spur formation or intimal proliferation, and subsequent venous thrombosis or venous stasis ulceration [2]. Because the iliac veins are not imaged with conventional lower extremity duplex ultrasound, these lesions can be frequently missed, even in symptomatic patients. Therefore, the diagnosis requires a high index of suspicion. The gold standard for diagnosis of MTS is intravascular ultrasound (IVUS), although CT angiography also has a high sensitivity and specificity for MTS in comparison to other imaging studies [3, 4]. Interventions include thrombectomy or thrombolysis, although stenting is often required to prevent recurrent thrombosis [5].
In renal transplant recipients with known MTS, the transplanted kidney is placed in the right iliac fossa to avoid venous outflow obstruction and subsequent graft compromise [6]. For patients with iliac vein or renal vein thrombosis after kidney transplantation, endovascular interventions with thrombectomy and stenting are both safe and successful, with preservation of allograft function over long-term followup [7, 8]. Simultaneous pancreas-kidney (SPK) transplantation utilizes the bilateral iliac fossa for vascular reconstruction, with pancreatic venous anastomoses to the right common iliac vein or IVC, and renal venous anastomoses to the left common iliac vein. There are no current guidelines for management of patients with undergoing SPK transplant with pre-operative or post-operative symptomatic MTS.
CASE PRESENTATION
A 57-year-old African-American woman with end-stage renal disease (Cr 6.7), secondary to diabetic nephropathy (HbA1C 8.2) underwent a SPK transplant with thymoglobulin induction. She had no prior history of venous thromboembolic events and no personal or family history of clotting disorders. Her pre-operative venous duplex was negative for deep vein thrombosis. Intra-operatively, the vascular reconstruction was performed in the standard configuration for this institution. The renal vein was anastomosed to the left external iliac vein, and the pancreatic portal vein was anastomosed to the IVC. (Fig. 1) She was eventually discharged with excellent kidney and pancreas allograft function (Cr 1.1, HbA1C 5.3).
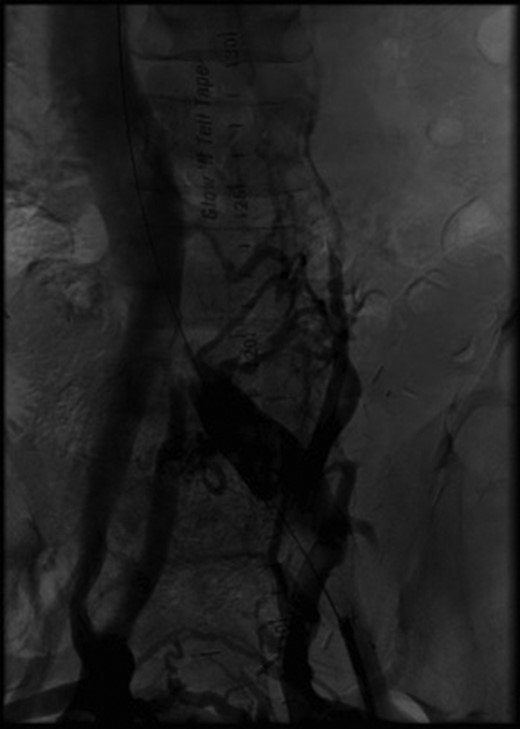
Initial angiography demonstrating thrombus in left common iliac vein with surrounding collaterals.
Two months after her transplant she developed abdominal pain and a CT scan was performed. Imaging demonstrated an extensive thrombus in the left common and external iliac veins, with compression of the left common iliac vein superior to the thrombus (Fig. 2). An interventional cardiology team performed angiography which showed a 100% occlusive lesion in the left CIV, with thrombotic defect in the left EIV, and patent pancreatic SMV and left renal vein. Balloon angioplasty and combined Angiojet and manual thrombectomy of the left common and external iliac veins was performed. A 16 mm x 40 mm Wallstent self-expanding metal stent was deployed in the left CIV, with a proximal landing site in the IVC, just distal to the pancreatic vein graft. Post-procedure angiography demonstrated a 30% residual stenosis, with patent flow through the LCIV and LEIV, and both the pancreatic and renal grafts. She was started on therapeutic anticoagulation after the procedure. Surveillance imaging 6-months post-procedure demonstrated no residual clot burden and appropriate stent positioning. Labs at 1 year follow up show persevered allograft function, with creatinine 0.91, amylase 88 and lipase 140, and no insulin requirements.
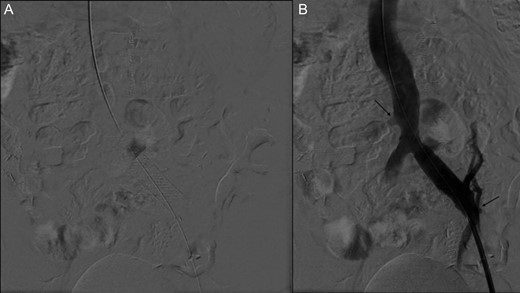
(A) Stent placement (B) Angiography post-thrombectomy and stent showing intact runoff to pancreatic and renal allografts (arrows).
DISCUSSION
Thrombectomy alone in MTS carries a high rate of thrombosis recurrence [9]. Therefore, these patients are managed through thrombectomy or thrombolysis, with subsequent stenting, as per current guidelines by both the Society of Interventional Radiology [9]. Stenting has significantly higher post-intervention patency rates, with nearly 80% patency at 5 years.
Prior case reports have documented that successful angiographic interventions and stenting can be performed on renal vein thromboses in kidney transplant patients without jeopardizing the graft [5]. Endovascular stenting of the LCIV and IVC after SPK transplant is particularly challenging because of the complex anatomy. Both the proximal and distal landing sites are restricted by allograft venous anastomoses, which if covered, would be fatal to the respective graft. Yet the stent must have an adequate length of purchase within each vessel to prevent stent migration.
Given the higher patency rates of bare metal stents, we elected to place a stainless steal stent and minimize the risk of rethrombosis. Our interventional cardiology team was able to both deploy the stent with adequate precision to preserve venous outflow from both grafts, and seat it appropriately to prevent movement. To our knowledge, this procedure has not been performed in a patient after SPK
In patients with known MTS prior to SPK, we recommend performing the portal vein anastomosis to the right CIV as this decreases the complexity if stent placement is ultimately needed. Alternatively, Fridell et al. describe a series of 49 patients who underwent simultaneous ipsilateral pancreas and kidney transplants, with pancreatic Y-graft and portal vein anastomoses to right common iliac artery and vein respectively, and renal artery and vein anastomosis to external iliac artery and vein respectively. They report comparable rates of pancreas (92%) and kidney (94%) allograft survival, as well as equivalent re-laparotomy rates (16%) [10]. This ipsilateral vascular configuration could offer an alternative reconstruction option for patients diagnosed with MTS prior to transplantation.
There is no literature on prophylactic stent placement for MTS in pre-operative transplant patients, and this is an area for future research. Our case demonstrates the need to maintain a high index of suspicion for the development of MTS when operating on the left iliac vessels. On retrospective review of her pre-operative CT scan, May-Thurner anatomy was identified with compression of the left CIV. Additionally, during the initial case, bleeding was noted from what was thought to be side branches of the IVC. Upon review of her case, this was more likely collateral veins in the retroperitoneum. Nevertheless, if patients develop symptomatic MTS post-operatively, our case demonstrates that thrombectomy and stenting can safely be performed using an endovascular approach to maintain allograft function. Our case also highlights the multi-disciplinary management of this complex case.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- angiogram
- stents
- thrombosis
- diabetes mellitus
- renal transplantation
- kidney failure, chronic
- follow-up
- reconstructive surgical procedures
- thrombectomy
- diagnostic imaging
- guidelines
- kidney
- pancreas
- transplantation
- thrombus
- may-thurner syndrome
- renal and pancreas transplantation
- common iliac artery
- allografting
- mohr-tranebjaerg syndrome
- anatomic variant
- iliac vein thrombosis
- endovascular procedures
- common iliac vein



